
| Home | Sources Directory | News Releases | Calendar | Articles | | Contact | |
Starch
| Starch | |
|---|---|
 |
|
| Identifiers | |
| CAS number | 9005-25-8 |
| EC-number | 232-679-6 |
| RTECS number | GM5090000 |
| Properties | |
| Molecular formula | (C6H10O5)n |
| Appearance | white powder |
| Density | 1.5 g/cm3 |
| Melting point |
decomp. |
| Solubility in water | none |
| Hazards | |
| MSDS | ICSC 1553 |
| EU Index | not listed |
| Autoignition temperature |
410 –C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 –C, 100 kPa) |
|
| Infobox references | |

| Look up starch in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to: Starch |
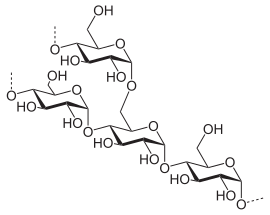
Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store. It is the most important carbohydrate in the human diet and is contained in such staple foods as potatoes, wheat, maize (corn), rice, and cassava.
Pure starch is a white, tasteless and odorless powder that is insoluble in cold water or alcohol. It consists of two types of molecules: the linear and helical amylose and the branched amylopectin. Depending on the plant, starch generally contains 20 to 25% amylose and 75 to 80% amylopectin.[1] Glycogen, the glucose store of animals, is a more branched version of amylopectin.
Starch is processed to produce many of the sugars in processed foods. When dissolved in warm water, it can be used as a thickening, stiffening or gluing agent, giving wheatpaste.
Contents |
[edit] Name
The word "starch" is derived from Middle English sterchen, meaning to stiffen. "Amylum" is Latin for starch, from the Greek "amulon" which means "not ground at a mill". The root amyl is used in biochemistry for several compounds related to starch.
[edit] History
Wheat starch paste was used by Egyptians to stiffen cloth and during weaving linen and possibly to glue papyrus.[2] Romans used it also in cosmetic creams, to powder the hair and to thicken sauces. Persians and Indians used it to make dishes similar to gothumai wheat halva. In China, with the invention of paper, rice starch was used as a surface treatment of the paper.
[edit] Energy store of plants
In photosynthesis, plants use light energy to produce glucose from carbon dioxide. The glucose is stored mainly in the form of starch granules, in plastids such as chloroplasts and especially amyloplasts. Toward the end of the growing season, starch accumulates in twigs of trees near the buds. Fruit, seeds, rhizomes, and tubers store starch to prepare for the next growing season.
Glucose is soluble in water, hydrophilic, binds much water and then takes up much space; glucose in the form of starch, on the other hand, is not soluble and can be stored much more compactly.
Glucose molecules are bound in starch by the easily hydrolyzed alpha bonds. The same type of bond can also be seen in the animal reserve polysaccharide glycogen. This is in contrast to many structural polysaccharides such as chitin, cellulose and peptidoglycan, which are bound by beta-bonds and are much more resistant to hydrolysis.
[edit] Biosynthesis
Plants produce starch by first converting glucose 1-phosphate to ADP-glucose using the enzyme glucose-1-phosphate adenylyltransferase. This step requires energy in the form of ATP. The enzyme starch synthase then adds the ADP-glucose via a 1,4-alpha glycosidic bond to a growing chain of glucose residues, liberating ADP and creating amylose. Starch branching enzyme introduces 1,6-alpha glycosidic bonds between these chains, creating the branched amylopectin. The starch debranching enzyme isoamylase removes some of these branches. Several isoforms of these enzymes exist, leading to a highly complex synthesis process.[3]
While amylose was traditionally thought to be completely unbranched, it is now known that some of its molecules contain a few branch points.[4]
Glycogen and amylopectin have the same structure, but the former has about one branch point per ten 1,4-alpha bonds, compared to about one branch point per thirty 1,4-alpha bonds in amylopectin.[5] Another difference is that glycogen is synthesised from UDP-glucose while starch is synthesised from ADP-glucose.
[edit] Properties
[edit] Structure
Starch molecules arrange themselves in the plant in semi-crystalline granules. Each plant species has a unique starch granular size: rice starch is relatively small (about 2î�m) while potato starches have larger granules (up to 100î�m). Although in absolute mass only about one quarter of the starch granules in plants consist of amylose, there are about 150 times more amylose molecules than amylopectin molecules. Amylose is a much smaller molecule than amylopectin.
Starch becomes soluble in water when heated. The granules swell and burst, the semi-crystalline structure is lost and the smaller amylose molecules start leaching out of the granule, forming a network that holds water and increasing the mixture's viscosity. This process is called starch gelatinization. During cooking the starch becomes a paste and increases further in viscosity. During cooling or prolonged storage of the paste, the semi-crystalline structure partially recovers and the starch paste thickens, expelling water. This is mainly caused by the retrogradation of the amylose. This process is responsible for the hardening of bread or staling, and for the water layer on top of a starch gel (syneresis).
Some cultivated plant varieties have pure amylopectin starch without amylose, known as waxy starches. The most used is waxy maize, others are glutinous rice, waxy potato starch. Waxy starches have less retrogradation, resulting in a more stable paste. High amylose starch, amylomaize, is cultivated for the use of its gel strength.
[edit] Hydrolysis
The enzymes that break down or hydrolyze starch into the constituent sugars are known as amylases.
Alpha-amylases are found in plants and in animals. Human saliva is rich in amylase, and the pancreas also secretes the enzyme. Individuals from populations with a high-starch diet tend to have more amylase genes than those with low-starch diets[6]; chimpanzees have very few amylase genes[6]. It is possible that turning to a high-starch diet was a significant event in human evolution.[7]
Beta-amylase cuts starch into maltose units. This process is important in the digestion of starch and is also used in brewing, where the amylase from the skin of the seed grains is responsible for converting starch to maltose (Malting, Mashing).
[edit] Dextrinization
If starch is subjected to dry heat, it breaks down to form pyrodextrins, in a process known as dextrinization. Pyrodextrins are brown in color. This process is partially responsible for the browning of toasted bread.
[edit] Chemical tests
Iodine solution is used to test for starch; a dark blue color indicates the presence of starch. The details of this reaction are not yet fully known, but it is thought that the iodine (I3' and I5' ions) fit inside the coils of amylose, the charge transfers between the iodine and the starch, and the energy level spacings in the resulting complex correspond to the absorption spectrum in the visible light region. The strength of the resulting blue color depends on the amount of amylose present. Waxy starches with little or no amylose present will color red.
Starch indicator solution consisting of water, starch and iodine is often used in redox titrations: in the presence of an oxidizing agent the solution turns blue, in the presence of reducing agent the blue color disappears because triiodide (I3') ions break up into three iodide ions, disassembling the starch-iodine complex. A 0.3% w/w solution is the standard concentration for a starch indicator. It is made by adding 3 grams of soluble starch to 1 litre of heated water; the solution is cooled before use (starch-iodine complex becomes unstable at temperatures above 35 –C).
Microscopy of starch granules - Each species of plant has a unique shape of starch granules in granular size, shape and crystallisation pattern. Under the microscope, starch grains stained with iodine illuminated from behind with polarized light show a distinctive Maltese cross effect (also known as extinction cross and birefringence).
[edit] Starch as food
Starch is the most important carbohydrate in the human diet and is contained in many staple foods. The major sources of starch intake worldwide are rice, wheat, maize (corn), potatoes and cassava. Widely used prepared foods containing starch are bread, pancakes, cereals, noodles, pasta, porridge and tortilla.[8]
Depending on the local climate other starch sources are used for food, such as acorn, arrowroot, arracacha, banana, barley, breadfruit, buckwheat, canna, colacasia, katakuri, kudzu, malanga, millet, oat, oca, polynesian arrowroot, sago, sorghum, sweet potato, rye, taro, water chestnut and yams. Chestnuts and edible beans, such as favas, lentils, mung bean and peas, are also rich in starch.
Digestive enzymes have problems digesting crystalline structures. Raw starch will digest poorly in the duodenum and small intestine, while bacterial degradation will take place mainly in the colon. Resistant starch is starch that escapes digestion in the small intestine of healthy individuals. In order to increase the digestibility, starch is cooked. Hence, before humans started using fire, eating grains was not a very useful way to get energy.
[edit] Starch industry
The starch industry extracts and refines starches from seeds, roots and tubers, by wet grinding, washing, sieving and drying. Today, the main commercial refined starches are cornstarch, tapioca, wheat and potato starch. To a lesser extent, sources include rice, sweet potato, sago and mung bean. Historically, Florida arrowroot was also commercialized. Still starch is extracted from more than 50 types of plants.
Untreated starch requires heat to thicken or gelatinize. When a starch is pre-cooked, it can then be used to thicken instantly in cold water. This is referred to as a pregelatinized starch.
[edit] Starch sugars
Starch can be hydrolyzed into simpler carbohydrates by acids, various enzymes, or a combination of the two. The resulting fragments are known as dextrins. The extent of conversion is typically quantified by dextrose equivalent (DE), which is roughly the fraction of the glycosidic bonds in starch that have been broken.
These starch sugars are by far the most common starch based food ingredient and are used as sweetener in many drinks and foods. They include:
- Maltodextrin, a lightly hydrolyzed (DE 10'20) starch product used as a bland-tasting filler and thickener.
- Various glucose syrup / corn syrups (DE 30'70), viscous solutions used as sweeteners and thickeners in many kinds of processed foods.
- Dextrose (DE 100), commercial glucose, prepared by the complete hydrolysis of starch.
- High fructose syrup, made by treating dextrose solutions with the enzyme glucose isomerase, until a substantial fraction of the glucose has been converted to fructose. In the United States, high fructose corn syrup is the principal sweetener used in sweetened beverages because fructose has better handling characteristics, such as microbiological stability, and more consistent sweetness/flavor. High fructose corn syrup has the same sweetness as sugar.
- Sugar alcohols, such as maltitol, erythritol, sorbitol, mannitol and hydrogenated starch hydrolysate, are sweeteners made by reducing sugars.
[edit] Modified starches
A modified food starch is a starch that has been chemically modified to allow the starch to function properly under conditions frequently encountered during processing or storage, such as high heat, high shear, low pH, freeze/thaw and cooling.
The modified starches are E coded according to the International Numbering System for Food Additives (INS):[9]
- 1401 Acid-treated starch
- 1402 Alkaline-treated starch
- 1403 Bleached starch
- 1404 Oxidized starch
- 1405 Starches, enzyme-treated
- 1410 Monostarch phosphate
- 1412 Distarch phosphate
- 1413 Phosphated distarch phosphate
- 1414 Acetylated distarch phosphate
- 1420 Starch acetate
- 1422 Acetylated distarch adipate
- 1440 Hydroxypropyl starch
- 1442 Hydroxypropyl distarch phosphate
- 1443 Hydroxypropyl distarch glycerol
- 1450 Starch sodium octenyl succinate
- 1451 Acetylated oxidized starch
INS 1401, 1402, 1403 and 1405 are in the EU food ingredients without an E-number. Typical modified starches for technical applications are cationic starches, hydroxyethyl starch and carboxymethylated starches.
[edit] Use as food additive
As an additive for food processing, food starches are typically used as thickeners and stabilizers in foods such as puddings, custards, soups, sauces, gravies, pie fillings, and salad dressings, and to make noodles and pastas.
Gummed sweets such as jelly beans and wine gums are not manufactured using a mold in the conventional sense. A tray is filled with native starch and leveled. A positive mold is then pressed into the starch leaving an impression of 1000 or so jelly beans. The jelly mix is then poured into the impressions and put into a stove to set. This method greatly reduces the number of molds that must be manufactured.
In the pharmaceutical industry, starch is also used as an excipient, as tablet disintegrant or as binder.
[edit] Industrial applications
Papermaking is the largest non-food application for starches globally, consuming millions of metric tons annually. In a typical sheet of copy paper for instance, the starch content may be as high as 8%. Both chemically modified and unmodified starches are used in papermaking. In the wet part of the papermaking process, generally called the 'wet-end', the starches used are cationic and have a positive charge bound to the starch polymer. These starch derivatives associate with the anionic or negatively charged paper fibers / cellulose and inorganic fillers. Cationic starches together with other retention and internal sizing agent help to give the necessary strength properties to the paper web to be formed in the papermaking process (wet strength), and to provide strength to the final paper sheet (dry strength).
In the dry end of the papermaking process the paper web is rewetted with a starch based solution. The process is called surface sizing. Starches used have been chemically, or enzymatically depolymerized at the paper mill or by the starch industry (oxidized starch). The size - starch solutions are applied to the paper web by means of various mechanical presses (size press). Together with surface sizing agent the surface starches impart additional strength to the paper web and additionally provide water hold out or 'size' for superior printing properties. Starch is also used in paper coating as one of the binders for the coating formulation a mixture of pigments, binders and thickeners. Coated paper has improved smoothness, hardness, whiteness and gloss and thus improves printing characteristics.
Corrugated board adhesives are the next largest application of non-food starches globally. Starch glues are mostly based on unmodified native starches, plus some additive such as borax and caustic soda. Part of the starch is gelatinized to carry the slurry of uncooked starches and prevent sedimentation. This opaque glue is called a SteinHall adhesive. The glue is applied on tips of the fluting. The fluted paper is pressed to paper called liner. This is then dried under high heat, which causes the rest of the uncooked starch in glue to swell/gelatinize. This gelatinizing makes the glue a fast and strong adhesive for corrugated board production.
Another large non-food starch application is in the construction industry, where starch is used in the gypsum wall board manufacturing process. Chemically modified or unmodified starches are added to the stucco containing primarily gypsum. Top and bottom heavyweight sheets of paper are applied to the formulation, and the process is allowed to heat and cure to form the eventual rigid wall board. The starches act as a glue for the cured gypsum rock with the paper covering, and also provide rigidity to the board.
Starch is used in the manufacture of various adhesives or glues[10] for book-binding, wallpaper adhesives, paper sack production, tube winding, gummed paper, envelop adhesives, school glues and bottle labeling.
Starch derivatives, such as yellow dextrins, can be modified by addition of some chemicals to form a hard glue for paper work; some of those forms use borax or soda ash, which are mixed with the starch solution at 50-70 –C to create a very good adhesive. Sodium silicate can be added to reinforce these formulae.
Clothing starch or laundry starch is a liquid that is prepared by mixing a vegetable starch in water (earlier preparations also had to be boiled), and is used in the laundering of clothes. Starch was widely used in Europe in the 16th and 17th centuries to stiffen the wide collars and ruffs of fine linen which surrounded the necks of the well-to-do. During the 19th century and early 20th century, it was stylish to stiffen the collars and sleeves of men's shirts and the ruffles of girls' petticoats by applying starch to them as the clean clothes were being ironed. Aside from the smooth, crisp edges it gave to clothing, it served practical purposes as well. Dirt and sweat from a person's neck and wrists would stick to the starch rather than to the fibers of the clothing, and would easily wash away along with the starch. After each laundering, the starch would be reapplied. Today, the product is sold in aerosol cans for home use.
Starch is also used to make some packing peanuts, and some drop ceiling tiles.
Textile chemicals from starch are used to reduce breaking of yarns during weaving; the warp yarns are sized, especially for cotton. Starch is also used as textile printing thickener.
In the printing industry, food grade starch[11] is used in the manufacture of anti-set-off spray powder used to separate printed sheets of paper to avoid wet ink being set off.
Starch is used to produce various bioplastics, synthetic polymers that are biodegradable. An example is polylactic acid.
For body powder, powdered corn starch is used as a substitute for talcum powder, and similarly in other health and beauty products.
In oil exploration, starch is used to adjust the viscosity of drilling fluid, which is used to lubricate the drill head and suspend the grinding residue in petroleum extraction.
Glucose from starch can be further fermented to biofuel ethanol.
Hydrogen production can use starch as the raw material, using enzymes.[12]
[edit] See also
- Modified starch
- Yeast extract
- Flour
- Distilled beverage, brewing from starch alcohol
- Baking, making starches digestible
- Cooking
- Acrylamide, present in fried potatoes
- Non-Newtonian fluid
[edit] References
- ^ Brown, W. H.; Poon, T. (2005). Introduction to organic chemistry (3rd ed.). Wiley. ISBN 0-471-44451-0..
- ^ Pliny the Elder, The Natural History (Pliny), Book XIII, Chapter 26, The paste used in preparation of paper
- ^ Smith, A M (2001). "The biosynthesis of starch granules". Biomacromolecules 2 (2): 335'41. doi:10.1021/bm000133c. PMID 11749190.
- ^ David R. Lineback, "Starch", in AccessScience@McGraw-Hill.
- ^ Stryer, Lubert; Berg, Jeremy Mark; Tymoczko, John L. (2002). "Section 11.2.2". Biochemistry (5th ed.). San Francisco: W.H. Freeman. ISBN 0-7167-3051-0. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=stryer.section.1517#1522.
- ^ a b "Diet and the evolution of human amylase gene copy number variation". Nature Genetics by Nature Publishing Group. http://www.nature.com/ng/journal/v39/n10/full/ng2123.html.
- ^ PZ Myers, Amylase and human evolution, December 11, 2008.
- ^ Anne-Charlotte Eliasson (2004). Starch in food: Structure, function and applications. Woodhead Publishing. ISBN 9780849325557.
- ^ Modified Starches. CODEX ALIMENTARIUS published in FNP 52 Add 9 (2001)
- ^ "Starch based glue". - ICI. http://www.nationalstarch.com/NationalStarch/About+Us/Our+Businesses.
- ^ "Spray Powder". Russell-Webb. http://www.russell-webb.com/anti_set_off_powder/soluble_anti-set-off-powder.html. Retrieved 2007-07-05.
- ^ Zhang YH, Evans BR, Mielenz JR, Hopkins RC, Adams MW (2007). "High-yield hydrogen production from starch and water by a synthetic enzymatic pathway". PLoS ONE 2 (5): e456. doi:10.1371/journal.pone.0000456. PMID 17520015. PMC 1866174. http://www.plosone.org/article/fetchArticle.action?articleURI=info:doi/10.1371/journal.pone.0000456.
[edit] External links
- European Association of starch manufactures AAF
- Starch, by Martin Chaplin
- Starch - Stärke, scientific journal on starch
|
|||||||||||||||||||||||||||||||||||||||||||||
|
SOURCES.COM is an online portal and directory for journalists, news media, researchers and anyone seeking experts, spokespersons, and reliable information resources. Use SOURCES.COM to find experts, media contacts, news releases, background information, scientists, officials, speakers, newsmakers, spokespeople, talk show guests, story ideas, research studies, databases, universities, associations and NGOs, businesses, government spokespeople. Indexing and search applications by Ulli Diemer and Chris DeFreitas.
For information about being included in SOURCES as a expert or spokesperson see the FAQ . For partnerships, content and applications, and domain name opportunities contact us.


