| Home | Sources Directory | News Releases | Calendar | Articles | | Contact | |
Antibiotic
In common usage, an antibiotic (from the Ancient Greek: á��î��„î� ' anti, "against", and î�î�î¿ς ' bios, "life") is a substance or compound that kills bacteria or inhibits their growth.[1] Antibiotics belong to the broader group of antimicrobial compounds, used to treat infections caused by microorganisms, including fungi and protozoa.
The term "antibiotic" was coined by Selman Waksman in 1942 to describe any substance produced by a microorganism that is antagonistic to the growth of other microorganisms in high dilution.[2] This original definition excluded naturally occurring substances that kill bacteria but are not produced by microorganisms (such as gastric juice and hydrogen peroxide) and also excluded synthetic antibacterial compounds such as the sulfonamides. Many antibiotics are relatively small molecules with a molecular weight less than 2000 Da.[citation needed]
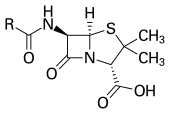
With advances in medicinal chemistry, most antibiotics are now semisynthetic'modified chemically from original compounds found in nature,[3] as is the case with beta-lactams (which include the penicillins, produced by fungi in the genus Penicillium, the cephalosporins, and the carbapenems). Some antibiotics are still produced and isolated from living organisms, such as the aminoglycosides, and others have been created through purely synthetic means: the sulfonamides, the quinolones, and the oxazolidinones. In addition to this origin-based classification into natural, semisynthetic, and synthetic, antibiotics may be divided into two broad groups according to their effect on microorganisms: Those that kill bacteria are bactericidal agents, whereas those that only impair bacterial growth are known as bacteriostatic agents.
Contents |
[edit] History of antibiotics
|
|
This article may be confusing or unclear to readers. Please help clarify the article; suggestions may be found on the talk page. (January 2010) |
Many treatments for infections prior to the beginning of the twentieth century were based on medicinal folklore. Treatments for infection in ancient Chinese medicine using plants with antimicrobial properties were described over 2,500 years ago.[4][5] Many other ancient cultures, including the ancient Egyptians and ancient Greeks used molds and plants to treat infections.[6][7] The discovery of the natural antibiotics produced by microorganisms stemmed from earlier work on the observation of antibiosis between micro-organisms. Louis Pasteur observed that, "if we could intervene in the antagonism observed between some bacteria, it would offer 'perhaps the greatest hopes for therapeutics'".[8] Synthetic antibiotic chemotherapy as a science and the story of antibiotic development began in Germany with Paul Ehrlich, a German medical scientist in the late 1880s.[9] Scientific endeavours to understand the science behind what caused these diseases, the development of synthetic antibiotic chemotherapy, the isolation of the natural antibiotics marked milestones in antibiotic development.[10]
Originally known as antibiosis, antibiotics were drugs which acted against bacteria. The term antibiosis, which means "against life," was introduced by the French bacteriologist Vuillemin as a descriptive name of the phenomenon exhibited by these drugs.[9] (Antibiosis was first described in 1877 in bacteria when Louis Pasteur and Robert Koch observed that an airborne bacillus could inhibit the growth of Bacillus anthracis.[11]). These drugs were later renamed antibiotics by Selman Waksman, an American microbiologist in 1942.[2][9]
Bacterial antagonism of Penicillium spp. were first described in England by John Tyndall in 1875.[8] The significance to antibiotic discovery was not realized until the work of Ehrlich on synthetic antibiotic chemotherapy, which marked the birth of the antibiotic revolution. Ehrlich noted that certain dyes would bind to and color human, animal, or bacterial cells, while others did not. He then extended the idea that it might be possible to make certain dyes or chemicals that would act as a magic bullet or selective drug that would bind to and kill bacteria while not harming the human host. After much experimentation, screening hundreds of dyes against various organisms, he discovered a medicinally useful drug, the man-made antibiotic, Salvarsan.[9][12][13] In 1928 Fleming made an important observation concerning the antibiosis by penicillin. Fleming postulated that the effect was mediated by a yet-unidentified antibiotic-like compound that could be exploited. Although he initially characterized some of its antibiotic properties, he did not pursue its development.[14][15] In the meantime, another synthetic antibacterial antibiotic Prontosil was developed and manufactured for commercial use by Domagk in 1932.[13] Prontosil, the first commercially available antibacterial antibiotic, was developed by a research team led by Gerhard Domagk (who received the 1939 Nobel Prize for Medicine for his efforts) at the Bayer Laboratories of the IG Farben conglomerate in Germany. Prontosil had a relatively broad effect against Gram-positive cocci but not against enterobacteria. The discovery and development of this first sulfonamide drug opened the era of antibiotics. In 1939, discovery by Rene Dubos of the first naturally derived antibiotic-like substance named gramicidin from B. brevis. It was one of the first commercially manufactured antibiotics in use during World War II to prove highly effective in treating wounds and ulcers.[16] Florey and Chain succeeded in purifying penicillin. The purified antibiotic displayed antibacterial activity against a wide range of bacteria. It also had low toxicity and could be taken without causing adverse effects. Furthermore, its activity was not inhibited by biological constituents such as pus, unlike the synthetic antibiotic class available at the time, the sulfonamides. The discovery of such a powerful antibiotic was unprecedented. The development of penicillin led to renewed interest in the search for antibiotic compounds with similar capabilities.[17] Because of their discovery of penicillin Ernst Chain, Howard Florey and Alexander Fleming shared the 1945 Nobel Prize in Medicine. Florey credited Dubos with pioneering the approach of deliberately, systematically searching for antibacterial compounds. Such a methodology had led to the discovery of gramicidin, which revived Florey's research in penicillin.[16]
[edit] Antimicrobial pharmacodynamics

The assessment of the activity of an antibiotic is crucial to the successful outcome of antimicrobial therapy. Non-microbiological factors such as host defense mechanisms, the location of an infection, the underlying disease as well as the intrinsic pharmacokinetic and pharmacodynamic properties of the antibiotic.[18] Fundamentally, antibiotics are classified as either having lethal (bactericidal) action against bacteria or are bacteriostatic, preventing bacterial growth. The bactericidal activity of antibiotics may be growth phase-dependent, and, in most but not all cases, the action of many bactericidal antibiotics requires ongoing cell activity and cell division for the drugs' killing activity.[19] These classifications are based on laboratory behavior; in practice, both of these are capable of ending a bacterial infection.[18][20]'In vitro' characterisation of the action of antibiotics to evaluate activity measure the minimum inhibitory concentration and minimum bactericidal concentration of an antimicrobial and are excellent indicators of antimicrobial potency.[21] However, in clinical practice, these measurements alone are insufficient to predict clinical outcome. By combining the pharmacokinetic profile of an antibiotic with the antimicrobial activity, several pharmacological parameters appear to be significant markers of drug efficacy.[22][23] The activity of antibiotics may be concentration-dependent and their characteristic antimicrobial activity increases with progressively higher antibiotic concentrations.[24] They may also be time-dependent, where their antimicrobial activity does not increase with increasing antibiotic concentrations; however, it is critical that a minimum inhibitory serum concentration is maintained for a certain length of time.[24] A laboratory evaluation of the killing kinetics of the antibiotic using kill curves is useful to determine the time- or concentration-dependence of .[18]
[edit] Antibiotic classes
Antibiotics are commonly classified based on their mechanism of action, chemical structure, or spectrum of activity. Most antibiotics target bacterial functions or growth processes.[9] Antibiotics that target the bacterial cell wall (penicillins, cephalosporins), or cell membrane (polymixins), or interfere with essential bacterial enzymes (quinolones, sulfonamides) are usually bactericidal in nature. Those that target protein synthesis, such as the aminoglycosides, macrolides, and tetracyclines, are usually bacteriostatic.[25] Further categorization is based on their target specificity: "Narrow-spectrum" antibiotics target particular types of bacteria, such as Gram-negative or Gram-positive bacteria, whereas broad-spectrum antibiotics affect a wide range of bacteria. In the last few years, three new classes of antibiotics have been brought into clinical use. This follows a 40-year hiatus in discovering new classes of antibiotic compounds. These new antibiotics are of the following three classes: cyclic lipopeptides (daptomycin), glycylcyclines (tigecycline), and oxazolidinones (linezolid). Tigecycline is a broad-spectrum antibiotic, whereas the two others are used for Gram-positive infections. These developments show promise as a means to counteract the bacterial resistance to existing antibiotics.
[edit] Production
Since the first pioneering efforts of Florey and Chain in 1939, the importance of antibiotics to medicine has led to much research into discovering and producing them. The process of production usually involves the screening of wide ranges of microorganisms, and their testing and modification. Production is carried out using fermentation, usually in strongly aerobic form.
[edit] Administration
Oral antibiotics are simply ingested, while intravenous antibiotics are used in more serious cases, such as deep-seated systemic infections. Antibiotics may also sometimes be administered topically, as with eye drops or ointments.
[edit] Side effects
Although antibiotics are, in general, considered safe and well-tolerated, they have been associated with a wide range of adverse effects.[26] Side-effects are many and varied, and can be very serious depending on the antibiotics used and the microbial organisms targeted. The safety profiles of newer medications may not be as well established as those that have been in use for many years.[26] Adverse effects can range from fever and nausea to major allergic reactions including photodermatitis and anaphylaxis.[citation needed] One of the more common side-effects is diarrhea, sometimes caused by the anaerobic bacterium Clostridium difficile, which results from the antibiotic's disrupting the normal balance of the intestinal flora,[27] Such overgrowth of pathogenic bacteria may be alleviated by ingesting probiotics during a course of antibiotics.[citation needed] An antibiotic-induced disruption of the population of the bacteria normally present as constituents of the normal vaginal flora may also occur, and may lead to overgrowth of yeast species of the genus Candida in the vulvo-vaginal area.[28] Other side-effects can result from interaction with other drugs, such as elevated risk of tendon damage from administration of a quinolone antibiotic with a systemic corticosteroid. Certain antibiotics administered by IV (e.g.aminoglycosides, vancomycin) can cause significant permanent hearing loss. [29]
[edit] Drug-drug interactions
[edit] Contraceptive pills
It has been hypothesized that interference of some antibiotics with the efficiency of birth control pills is thought to occur in two ways. Modification of the intestinal flora may result in reduced absorption of estrogens. Second, induction of hepatic liver enzymes causing them to metabolize the pill's active ingredients faster may affect the pill's usefulness.[30] However, the majority of studies indicate that antibiotics do not interfere with contraception.[30] Even though a small percentage of women may experience decreased effectiveness of birth control pills while taking an antibiotic, the failure rate is comparable to the failure rate of those taking the pill.[31] Moreover, there have been no studies that have conclusively demonstrated that disruption of the gut flora affects contraception.[32][33] Interaction with the combined oral contraceptive pill through induction of hepatic enzymes by the broad-spectrum antibiotic rifampicin has been shown to occur. It is recommended that extra contraceptive measures are applied during antimicrobial therapy using these antimicrobials.[30]
[edit] Alcohol
Interactions between alcohol and antibiotics vary depending on the specific antibiotic, and, in some cases, can cause severe side-effects and decrease effectiveness.
- "It is sensible to avoid drinking alcohol when taking medication. However, it is unlikely that drinking alcohol in moderation will cause problems if you are taking most "common" antibiotics." However, there are specific types of antibiotics with which alcohol should be avoided completely, because of serious side-effects.[34]
Because of the risks of side-effects and effectiveness, one should check the specific indications on the specific antibiotic, but there is no categorical danger in mixing alcohol and [some] antibiotics. Despite the lack of a categorical counterindication, the belief that alcohol and antibiotics should never be mixed is widespread, as indicated in a survey in one British clinic.
- "[P]atients often assume that they should avoid alcohol when taking any antibiotics. ...this belief has no foundation."[35]
One potential source of the myth is from STD clinics in the 1950s and 1960s.[36] Doctors gave the advice for moral reasons as they were worried that alcohol would reduce the inhibitions of sufferers and lead to further spread of diseases such as gonorrhoea.[37] It has been suggested, but not corroborated, that the origin of this myth centers on the fact that, during World War II, penicillin was in short supply and was recycled from urine; convalescing soldiers that drank beer produced a greater volume of urine, and, thus, were banned from drinking beer, leading to the belief that alcohol interacted poorly with antibiotics.[35]
[edit] Specific effects
By way of side-effects, certain antibiotics, including metronidazole, tinidazole, cephamandole, latamoxef, cefoperazone, cefmenoxime, and furazolidone, cause a disulfiram-like chemical reaction with alcohol by inhibiting metabolism by acetaldehyde dehydrogenase, leading to serious side-effects, which include severe vomiting, nausea, and shortness of breath. Alcohol consumption while taking such antibiotics is, therefore, prohibited.[34]
Other effects of alcohol involve the activity of liver enzymes, which break down the antibiotics.[38] In addition, serum levels of doxycycline and erythromycin succinate[clarification needed] may, in certain circumstances, be significantly reduced by alcohol consumption.[39] This is particularly important, since these drugs are bacteriostatic and require a sustained level of the drug in the body to be effective: Increased metabolism and clearance would result in diminished pharmacotherapeutic effect.
Alcohol can interfere with the activity or metabolization of antibiotics [40]
[edit] Antibiotic resistance
The emergence of antibiotic resistance is an evolutionary process that is based on selection for organisms that have enhanced ability to survive doses of antibiotics that would have previously been lethal.[41] Antibiotics like Penicillin and Erythromycin, which used to be one-time miracle cures are now less effective because bacteria have become more resistant.[42] Antibiotics themselves act as a selective pressure that allows the growth of resistant bacteria within a population and inhibits susceptible bacteria.[43] Antibiotic selection of pre-existing antibiotic resistant mutants within bacterial populations was demonstrated in 1943 by the Luria'Delbrück experiment.[44] Survival of bacteria often results from an inheritable resistance.[45] Any antibiotic resistance may impose a biological cost. Spread of antibiotic-resistant bacteria may be hampered by reduced fitness associated with the resistance, which is disadvantageous for survival of the bacteria when antibiotic is not present. Additional mutations, however, may compensate for this fitness cost and aids the survival of these bacteria.[46]
The underlying molecular mechanisms leading to antibiotic resistance can vary. Intrinsic resistance may naturally occur as a result of the bacteria's genetic makeup.[47] The bacterial chromosome may fail to encode a protein that the antibiotic targets. Acquired resistance results from a mutation in the bacterial chromosome or the acquisition of extra-chromosomal DNA.[47] Antibiotic-producing bacteria have evolved resistance mechanisms that have been shown to be similar to, and may have been transferred to, antibiotic-resistant strains.[48][49] The spread of antibiotic resistance mechanisms occurs through vertical transmission of inherited mutations from previous generations and genetic recombination of DNA by horizontal genetic exchange.[45] Antibiotic resistance is exchanged between different bacteria by plasmids that carry genes that encode antibiotic resistance that may result in co-resistance to multiple antibiotics.[45][50] These plasmids can carry different genes with diverse resistance mechanisms to unrelated antibiotics but because they are located on the same plasmid multiple antibiotic resistance to more than one antibiotic is transferred.[50] On the other hand, cross-resistance to other antibiotics within the bacteria results when the same resistance mechanism is responsible for resistance to more than one antibiotic is selected for.[50]
Antibiotic-resistant microorganisms, sometimes referred to as "superbugs", may contribute to the re-emergence of diseases which are currently well-controlled. For example, cases of tuberculosis (TB) that are resistant to traditionally effective treatments remain a cause of great concern to health professionals. Every year, nearly half a million new cases of multidrug-resistant tuberculosis (MDR-TB) are estimated to occur worldwide.[51] NDM-1 is a newly-identified enzyme that makes bacteria resistant to a broad range of beta-lactam antibiotics.[52] United Kingdom Health Protection Agency has stated that "most isolates with NDM-1 enzyme are resistant to all standard intravenous antibiotics for treatment of severe infections."[53]
[edit] Antibiotic misuse
The first rule of antibiotics is try not to use them, and the second rule is try not to use too many of them.[54]'Paul L. Marino, The ICU Book
Inappropriate antibiotic treatment and overuse of antibiotics have been a contributing factor to the emergence of resistant bacteria. The problem is further exacerbated by self-prescribing of antibiotics by individuals without the guidelines of a qualified clinician and the non-therapeutic use of antibiotics as growth promoters in agriculture.[55] Antibiotics are frequently prescribed for indications in which their use is not warranted, an incorrect or sub-optimal antibiotic is prescribed or in some cases for infections likely to resolve without treatment.[26][55] The overuse of antibiotics like penicillin and erythromycin, which used to be one-time miracle cures, were associated with emerging resistance since the 1950s.[42][56] Therapeutic usage of antibiotics in hospitals has been seen to be associated with increases in multi-antibiotic-resistant bacteria.[56]
Common forms of antibiotic misuse include excessive use of prophylactic antibiotics in travelers, failure to take into account the patient's weight and history of prior antibiotic use when prescribing, since both can strongly affect the efficacy of an antibiotic prescription, failure to take the entire prescribed course of the antibiotic, failure to prescribe or take the course of treatment at fairly precise correct daily intervals (e.g., "every 8 hours" rather than merely "3x per day"), or failure to rest for sufficient recovery to allow clearance of the infecting organism. These practices may facilitate the development of bacterial populations with antibiotic resistance. Inappropriate antibiotic treatment is another common form of antibiotic misuse. A common example is the prescription and use of antibiotics to treat viral infections such as the common cold that have no effect. One study on respiratory tract infections found "physicians were more likely to prescribe antibiotics to patients who they believed expected them, although they correctly identified only about 1 in 4 of those patients".[57] Multifactorial interventions aimed at both physicians and patients can reduce inappropriate prescribing of antibiotics.[58] Delaying antibiotics for 48 hours while observing for spontaneous resolution of respiratory tract infections may reduce antibiotic usage; however, this strategy may reduce patient satisfaction.[59]
Several organizations concerned with antimicrobial resistance are lobbying to improve the regulatory climate.[55] Approaches to tackling the issues of misuse and overuse of antibiotics by the establishment of the U.S. Interagency Task Force on Antimicrobial Resistance, which aims to actively address the problem antimicrobial resistance, are being organised and coordinated by the US Centers for Disease Control and Prevention, the Food and Drug Administration (FDA), and the National Institutes of Health (NIH), as well as other federal agencies.[60] An NGO campaign group is Keep Antibiotics Working.[61] In France, an "Antibiotics are not automatic" government campaign starting in 2002 led to a marked reduction of unnecessary antibiotic prescriptions, especially in children.[62] In the United Kingdom, there are NHS posters in many doctors' surgeries indicating that 'no amount of antibiotics will get rid of your cold', with many patients specifically requesting antibiotics from their doctor inappropriately, believing they treat viral infections.
In agriculture, associated antibiotic resistance with the non-therapeutic use of antibiotics as growth promoters in animals resulted in their restricted use in the UK in the 1970 (Swann report 1969). At the current time, there is a EU-wide ban on the non-therapeutic use of antibiotics as growth promoters. It is estimated that greater than 70% of the antibiotics used in U.S. are given to feed animals (e.g., chickens, pigs, and cattle) in the absence of disease.[63] Antibiotic use in food animal production has been associated with the emergence of antibiotic-resistant strains of bacteria including Salmonella spp., Campylobacter spp., Escherichia coli, and Enterococcus spp.[64][65] Evidence from some US and European studies suggest that these resistant bacteria cause infections in humans that do not respond to commonly prescribed antibiotics. In response to these practices and attendant problems, several organizations (e.g., The American Society for Microbiology (ASM), American Public Health Association (APHA) and the American Medical Association (AMA)) have called for restrictions on antibiotic use in food animal production and an end to all non-therapeutic uses.[citation needed] However, delays in regulatory and legislative actions to limit the use of antibiotics are common, and may include resistance to these changes by industries using or selling antibiotics, as well as time spent on research to establish causal links between antibiotic use and emergence of untreatable bacterial diseases. Two federal bills (S.742[66] and H.R. 2562[67]) aimed at phasing out non-therapeutic antibiotics in US food animal production were proposed but not passed.[66][67] These bills were endorsed by public health and medical organizations including the American Holistic Nurses' Association, the American Medical Association, and the American Public Health Association (APHA).[68] The EU has banned the use of antibiotics as growth promotional agents since 2003.[69]
[edit] Beyond antibiotics: Treating multi-drug-resistant bacteria
Multi-drug-resistant organisms (MDRO), in general, refer to bacteria that are not affected by the clinical doses of classical antibiotics, in particular, the antibiotics used to treat them until recently. The rise of these organisms has created a need for alternative antibacterial therapies.
[edit] Resistance-modifying agents
One solution to combat resistance currently being researched is the development of pharmaceutical compounds that would revert multiple antibiotic resistance. These so-called resistance-modifying agents may target and inhibit MDR mechanisms, rendering the bacteria susceptible to antibiotics to which they were previously resistant. These compounds' targets include among others
- Efflux inhibition(Phe-Arg-î�-naphthylamide)[70]
- Beta-Lactamase inhibitors - Including Clavulanic acid and Sulbactam
[edit] Phage Therapy
Phage therapy, the use of a particular group of viruses capable of invading bacteria known as phages to treat bacterial infections.[71][72] Phage are commonly a part of the ecology surrounding bacteria and provide substantial population control of bacteria in the intestine, the ocean, the soil, and other environments.[73] The therapy was in use during the 1920s and 1930s on humans in the US, Western Europe, and Eastern Europe. The success of these therapies is largely anecdotal, and rigorous scientific studies in the form of clinical trials commonly used to evaluate the efficacy of new medications on the efficacy of phage therapy are limited[72] The original publications into phage therapy are also generally inaccessible, even to persons with Russian language fluency. With the discovery of penicillin in the 1940s, Europe and the US abandoned research into phage therapy and began work to develop antibiotics as a therapeutic strategy combate bacterial infections. However, in the former Soviet Union, phage therapies continued to be studied in the Eliava Institute of Bacteriophage, Microbiology & Virology, Republic of Georgia.[74] With the development of antibiotic-resistant bacteria, there was a renewed interest in development of phage therapy as a viable alternative to antibiotic treatment of bacterial infection in western medicine.[75] Research is currently ongoing; companies (Intralytix, Novolytics, UK, Gangagen, India), universities, and foundations in North America and Europe are currently researching phage therapies.[75][76][77][78] However, concerns about genetic engineering in freely released viruses currently limit certain aspects of phage therapy. One result is attempts to use phage in ways other than to directly infect the bacteria.[79][80] While bacteriophage and related therapies provide a possible solution to aspects of antibiotic resistance, much more research is needed to realise their potential[72]
[edit] Bacteriocins
Bacteriocins are also a growing alternative to the classic small-molecule antibiotics.[81] Different classes of bacteriocins have different potential as therapeutic agents. Small molecule bacteriocins (microcins, for example, and lantibiotics) may be similar to the classic antibiotics; colicin-like bacteriocins are more likely to be narrow-spectrum, demanding new molecular diagnostics prior to therapy but also not raising the spectre of resistance to the same degree. One drawback to the large-molecule antibiotics is that they have relative difficulty crossing membranes and travelling systemically throughout the body. For this reason, they are most often proposed for application topically or gastrointestinally.[82] Because bacteriocins are peptides, they are more readily engineered than small molecules.[83] This may permit the generation of cocktails and dynamically improved antibiotics that are modified to overcome resistance.
[edit] Nutrient withdrawal
Nutrient withdrawal is a potential strategy for replacing or supplementing antibiotics. The restriction of iron availability is one way the human body limits bacterial proliferation.[84] Mechanisms for freeing iron from the body (such as toxins and siderophores) are common among pathogens. Building on this dynamic, various research groups are attempting to produce novel chelators that would withdraw iron otherwise available to pathogens (bacterial,[85] fungal [86] and parasitic [87]). This is distinct from chelation therapy for conditions other than bacterial infections - including successful treatment for iron overload.
[edit] Vaccines
Vaccines are a commonly suggested method for combating MDRO infections. They actually fit within a larger class of therapies that rely on immune modulation or augmentation. These therapies either excite or reinforce the natural immune competency of the infected or susceptible host, leading to the activity of macrophages, the production of antibodies, inflammation, or other classic immune reactions.
Just as the macrophage engulfs and consumes bacteria, various forms of biotherapy have been suggested that employ organisms to consume the pathogens. This includes the employment of protozoa [88] and maggot therapy.
[edit] Probiotics
Probiotics are another alternative that goes beyond traditional antibiotics by employing a live culture, which may, in theory, establish itself as a symbiont'competing, inhibiting, or simply interfering with colonization by pathogens.[89]
[edit] Beyond antibiotics: treating non-bacterial infections
|
|
This section needs attention from an expert on the subject. See the talk page for details. WikiProject Pharmacology or the Pharmacology Portal may be able to help recruit an expert. (July 2009) |
|
|
This section is missing citations or needs footnotes. Please help add inline citations to guard against copyright violations and factual inaccuracies. (October 2009) |
The comparative ease of identifying compounds that safely cured bacterial infections was more difficult to duplicate in treatments of fungal and viral infections. Antibiotic research led to great strides in the knowledge of biochemistry, establishing large differences between the cellular and molecular physiology of the bacterial cell and that of the mammalian cell. This explained the observation that many compounds that are toxic to bacteria are non-toxic to human cells. In contrast, the basic biochemistries of the fungal cell and the mammalian cell are much more similar. Most antifungal drugs have targeted steps in the synthesis of the fungal cell membrane (e.g., imidazole, triazole, and allylamine antifungals) or target components of the formed cell wall (e.g., polyene antifungals). However, toxicity is not fully avoided in the case of polyene antifungals since they can target human membrane cholesterol, mistaking it for fungal ergosterol.
This restricts the development and use of therapeutic compounds that attack a fungal cell, while not harming mammalian cells. Similar problems exist in antibiotic treatments of viral diseases. Human viral metabolic biochemistry is very closely similar to human biochemistry, and the possible targets of antiviral compounds are restricted to very few components unique to a mammalian virus.
For related articles, see fungicide, antifungal drug, and antiviral drug.
[edit] References
- ^ Davey PG (2000). "Antimicrobial chemotherapy". in Ledingham JGG, Warrell DA. Concise Oxford Textbook of Medicine. Oxford: Oxford University Press. p. 1475. ISBN 0192628704.
- ^ a b SA Waksman (1947). "What Is an Antibiotic or an Antibiotic Substance?". Mycologia 39 (5): 565'569. doi:10.2307/3755196. PMID 20264541. http://jstor.org/stable/3755196.
- ^ von Nussbaum F. et al. (2006). "Medicinal Chemistry of Antibacterial Natural Products ' Exodus or Revival?". Angew. Chem. Int. Ed. 45 (31): 5072'5129. doi:10.1002/anie.200600350. PMID 16881035.
- ^ Lindblad WJ (2008). "Considerations for Determining if a Natural Product Is an Effective Wound-Healing Agent". International Journal of Lower Extremity Wounds 7 (2): 75'81. doi:10.1177/1534734608316028. PMID 18483011.
- ^ How Products Are Made: Antibiotics
- ^ Forrest RD (March 1982). "Early history of wound treatment". J R Soc Med 75 (3): 198'205. PMID 7040656.
- ^ M. Wainwright (1989). "Moulds in ancient and more recent medicine" ([dead link]). Mycologist 3 (1): 21'23.. doi:10.1016/S0269-915X(89)80010-2. http://www.fungi4schools.org/Reprints/Mycologasdfdsfadsfasfdfasdfist_articles/Post-16/Medical/V03pp021-023folk_medicine.pdf.
- ^ a b Kingston W (June 2008). "Irish contributions to the origins of antibiotics". Irish journal of medical science 177 (2): 87'92. doi:10.1007/s11845-008-0139-x. PMID 18347757.
- ^ a b c d e Calderon CB, Sabundayo BP (2007). Antimicrobial Classifications: Drugs for Bugs. In Schwalbe R, Steele-Moore L, Goodwin AC. Antimicrobial Susceptibility Testing Protocols. CRC Press. Taylor & Frances group. ISBN 0-8247-4100-5
- ^ Foster W, Raoult A (December 1974). "Early descriptions of antibiosis". J R Coll Gen Pract 24 (149): 889'94. PMID 4618289.
- ^ H. Landsberg (1949). "Prelude to the discovery of penicillin". Isis 40 (3): 225'227.. doi:10.1086/349043.
- ^ Limbird LE (December 2004). "The receptor concept: a continuing evolution". Mol. Interv. 4 (6): 326'36. doi:10.1124/mi.4.6.6. PMID 15616162.
- ^ a b Bosch F, Rosich L (2008). "The contributions of Paul Ehrlich to pharmacology: a tribute on the occasion of the centenary of his Nobel Prize". Pharmacology 82 (3): 171'9. doi:10.1159/000149583. PMID 18679046.
- ^ Fleming A (1980). "Classics in infectious diseases: on the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae by Alexander Fleming, Reprinted from the British Journal of Experimental Pathology 10:226-236, 1929". Rev. Infect. Dis. 2 (1): 129'39. PMID 6994200. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2566493/.
- ^ Sykes R (2001). "Penicillin: from discovery to product". Bull. World Health Organ. 79 (8): 778'9. PMID 11545336.
- ^ a b Van Epps HL (2006). "René Dubos: unearthing antibiotics". J. Exp. Med. 203 (2): 259. doi:10.1084/jem.2032fta. PMID 16528813.
- ^ HW Florey (1945). "Use of Micro-organisms for therapeutic purposes". Br Med J. 2 (4427): 635'642. doi:10.1136/bmj.2.4427.635.
- ^ a b c Pankey GA, Sabath LD. (March 2004). "Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections.". Clin Infect Dis. 38 (6): 864'870. doi:10.1086/381972. PMID 14999632.
- ^ Mascio CT, Alder JD, Silverman JA (December 2007). "Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells". Antimicrob. Agents Chemother. 51 (12): 4255'60. doi:10.1128/AAC.00824-07. PMID 17923487.
- ^ Pelczar, M.J., Chan, E.C.S. and Krieg, N.R. (1999) 'Host-Parasite Interaction; Nonspecific Host Resistance', In: Microbiology Conceptsand Applications, 6th ed., McGraw-Hill Inc., New York, U.S.A. pp. 478-479.
- ^ Wiegand I, Hilpert K, Hancock REW (January 2008). "Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC)of antimicrobial substances". Nat Protoc. 3 (2): 163'175. doi:10.1038/nprot.2007.521. PMID 18274517.
- ^ Spanu T, Santangelo R, Andreotti F, Cascio GL, Velardi G, Fadda G (February 2004). "Antibiotic therapy for severe bacterial infections: correlation between the inhibitory quotient and outcome". Int. J. Antimicrob. Agents 23 (2): 120'8. doi:10.1016/j.ijantimicag.2003.06.006. PMID 15013036.
- ^ (accessed 13th Nov 2008)
- ^ a b Rhee KY, Gardiner DF (September 2004). "Clinical relevance of bacteriostatic versus bactericidal activity in the treatment of gram-positive bacterial infections". Clin. Infect. Dis. 39 (5): 755'6. doi:10.1086/422881. PMID 15356797.
- ^ Finberg RW, Moellering RC, Tally FP, et al. (November 2004). "The importance of bactericidal drugs: future directions in infectious disease". Clin. Infect. Dis. 39 (9): 1314'20. doi:10.1086/425009. PMID 15494908.
- ^ a b c Slama TG, Amin A, Brunton SA, et al. (July 2005). "A clinician's guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria". Am. J. Med. 118 Suppl 7A: 1S'6S. doi:10.1016/j.amjmed.2005.05.007. PMID 15993671.
- ^ University of Michigan Health System: Antibiotic-Associated Diarrhea, November 26, 2006
- ^ Pirotta MV, Garland SM (2006). "Genital Candida species detected in samples from women in Melbourne, Australia, before and after treatment with antibiotics". J Clin Microbiol. 44 (9): 3213'3217. doi:10.1128/JCM.00218-06. PMID 16954250.
- ^ Finegold, SM and Davis, A. Antibiotics and antibacterials, general, Calif Med. 1969 November; 111(5): 364'373.
- ^ a b c Weaver K, Glasier A (February 1999). "Interaction between broad-spectrum antibiotics and the combined oral contraceptive pill. A literature review". Contraception 59 (2): 71'8. doi:10.1016/S0010-7824(99)00009-8. PMID 10361620. http://linkinghub.elsevier.com/retrieve/pii/S0010-7824(99)00009-8.
- ^ Weisberg E (May 1999). "Interactions between oral contraceptives and antifungals/antibacterials. Is contraceptive failure the result?". Clin Pharmacokinet 36 (5): 309'13. doi:10.2165/00003088-199936050-00001. PMID 10384856.
- ^ Hassan T (March 1987). "Pharmacologic considerations for patients taking oral contraceptives". Conn Dent Stud J 7: 7'8. PMID 3155374.
- ^ Orme ML, Back DJ (December 1990). "Factors affecting the enterohepatic circulation of oral contraceptive steroids". Am. J. Obstet. Gynecol. 163 (6 Pt 2): 2146'52. PMID 2256523. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@rn+57-63-6.
- ^ a b "Can I drink alcohol while taking antibiotics?". NHS Direct (UK electronic health service). http://www.nhs.uk/chq/pages/871.aspx. Retrieved 2008-02-17.
- ^ a b Lwanga, J; Mears, A; Bingham, J S; Bradbeer, C S (16 December 2008). "Do antibiotics and alcohol mix? The beliefs of genitourinary clinic attendees". British Medical Journal 337: a2885. doi:10.1136/bmj.a2885BMJ 2008;337:a2885
- ^ Kruszelnicki, Karl S. (2005-06-02). "Alcohol and Antibiotics". ABC Science. http://www.abc.net.au/science/articles/2005/06/02/1380836.htm?site=science/greatmomentsinscience. Retrieved 2010-02-25.
- ^ Ferner, R.E. (September 1998). "You should know, you're a medic". http://archive.student.bmj.com/back_issues/0998/data/0998ed5.htm. Retrieved 2010-02-25.
- ^ "Antibiotics FAQ". McGill University, Canada. Archived from the original on February 16, 2008. http://web.archive.org/web/20080216195750/http://www.mcgill.ca/studenthealth/information/generalhealth/antibiotics/. Retrieved 2008-02-17.
- ^ Stockley, IH (2002), Stockley's Drug Interactions. 6th ed. London: Pharmaceutical Press.
- ^ "antibiotics-and-alcohol". http://www.mayoclinic.com/health/antibiotics-and-alcohol/AN01802., Mayo Clinic
- ^ Cowen LE (March 2008). "The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype". Nat. Rev. Microbiol. 6 (3): 187'98. doi:10.1038/nrmicro1835. PMID 18246082.
- ^ a b Pearson, Carol (2007-02-28). "Antibiotic Resistance Fast-Growing Problem Worldwide". Voice Of America. http://voanews.com/english/archive/2007-02/2007-02-28-voa33.cfm. Retrieved 2008-12-29.
- ^ Levy SB (October 1994). "Balancing the drug-resistance equation". Trends Microbiol. 2 (10): 341'2. doi:10.1016/0966-842X(94)90607-6. PMID 7850197.
- ^ Luria SE, Delbrück M (November 1943). "Mutations of Bacteria from Virus Sensitivity to Virus Resistance". Genetics 28 (6): 491'511. PMID 17247100. PMC 1209226. http://www.genetics.org/cgi/pmidlookup?view=long&pmid=17247100.
- ^ a b c Witte W (September 2004). "International dissemination of antibiotic resistant strains of bacterial pathogens". Infect. Genet. Evol. 4 (3): 187'91. doi:10.1016/j.meegid.2003.12.005. PMID 15450197.
- ^ Andersson DI (October 2006). "The biological cost of mutational antibiotic resistance: any practical conclusions?". Curr. Opin. Microbiol. 9 (5): 461'5. doi:10.1016/j.mib.2006.07.002. PMID 16890008.
- ^ a b Alekshun MN, Levy SB (March 2007). "Molecular mechanisms of antibacterial multidrug resistance". Cell 128 (6): 1037'50. doi:10.1016/j.cell.2007.03.004. PMID 17382878.
- ^ Marshall CG, Lessard IA, Park I, Wright GD (September 1998). "Glycopeptide antibiotic resistance genes in glycopeptide-producing organisms". Antimicrob. Agents Chemother. 42 (9): 2215'20. PMID 9736537. PMC 105782. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=9736537.
- ^ Nikaido H (February 2009). "Multidrug Resistance in Bacteria". Annu. Rev. Biochem. 78: 090220114451097. doi:10.1146/annurev.biochem.78.082907.145923. PMID 19231985.
- ^ a b c Baker-Austin C, Wright MS, Stepanauskas R, McArthur JV (April 2006). "Co-selection of antibiotic and metal resistance". Trends Microbiol. 14 (4): 176'82. doi:10.1016/j.tim.2006.02.006. PMID 16537105.
- ^ "Health ministers to accelerate efforts against drug-resistant TB". World Health Organization (WHO).
- ^ Boseley, Sarah (12 August 2010). "Are you ready for a world without antibiotics?". The Guardian.
- ^ "Health Protection Report". Health Protection Agency. 3 July 2009. http://www.hpa.org.uk/hpr/archives/2009/news2609.htm#ndm1.
- ^ Marino PL (2007). "Antimicrobial therapy". The ICU book. Hagerstown, MD: Lippincott Williams & Wilkins. p. 817. ISBN 0-7817-4802-X.
- ^ a b c Larson E (2007). "Community factors in the development of antibiotic resistance.". Annu Rev Public Health 28: 435'447. doi:10.1146/annurev.publhealth.28.021406.144020. PMID 17094768.
- ^ a b Hawkey PM (September 2008). "The growing burden of antimicrobial resistance". J. Antimicrob. Chemother. 62 Suppl 1: i1'9. doi:10.1093/jac/dkn241. PMID 18684701.
- ^ Ong S, Nakase J, Moran GJ, Karras DJ, Kuehnert MJ, Talan DA (2007). "Antibiotic use for emergency department patients with upper respiratory infections: prescribing practices, patient expectations, and patient satisfaction". Annals of emergency medicine 50 (3): 213'20. doi:10.1016/j.annemergmed.2007.03.026. PMID 17467120.
- ^ Metlay JP, Camargo CA, MacKenzie T, et al. (2007). "Cluster-randomized trial to improve antibiotic use for adults with acute respiratory infections treated in emergency departments". Annals of emergency medicine 50 (3): 221'30. doi:10.1016/j.annemergmed.2007.03.022. PMID 17509729.
- ^ Spurling G, Del Mar C, Dooley L, Foxlee R (2007). "Delayed antibiotics for respiratory infections". Cochrane database of systematic reviews (Online) (3): CD004417. doi:10.1002/14651858.CD004417.pub3. PMID 17636757.
- ^ "[1]." Centers for Disease Control and Prevention. Retrieved on March 12, 2009.
- ^ "Keep Antibiotics Working". Keep Antibiotics Working. http://www.keepantibioticsworking.com/new/index.cfm. Retrieved 2010-05-21.
- ^ Sabuncu E, David J, Bernède-Bauduin C et al. (2009). "Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002'2007". PLoS Med 6 (6): e1000084. doi:10.1371/journal.pmed.1000084. PMID 19492093. PMC 2683932. http://www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.1000084.
- ^ Mellon, M et al. (2001) Hogging It!: Estimates of Antimicrobial Abuse in Livestock, 1st ed. Cambridge, MA: Union of Concerned Scientists.
- ^ (accessed Nov 12, 2008)
- ^ (accessed Nov 12, 2008)
- ^ a b GovTrack.us. S. 742--109th Congress (2005): Preservation of Antibiotics for Medical Treatment Act of 2005, GovTrack.us (database of federal legislation) <http://www.govtrack.us/congress/bill.xpd?bill=s109-742> (accessed Nov 12, 2008)
- ^ a b GovTrack.us. H.R. 2562--109th Congress (2005): Preservation of Antibiotics for Medical Treatment Act of 2005, GovTrack.us (database of federal legislation) <http://www.govtrack.us/congress/bill.xpd?bill=h109-2562> (accessed Nov 12, 2008)
- ^ (accessed Nov 12, 2008)
- ^ (accessed Nov 12, 2008)
- ^ B. Marquez. (2005). Bacterial efflux systems and efflux pumps inhibitors. Biochimie87 1137'1147
- ^ Abedon ST, Calendar RL, ed (2005). The Bacteriophages.
- ^ a b c Mattey M, Spencer J (December 2008). "Bacteriophage therapy--cooked goose or phoenix rising?". Curr. Opin. Biotechnol. 19 (6): 608'12. doi:10.1016/j.copbio.2008.09.001. PMID 18926909.
- ^ Stephen T. Abedon (Editor) (2008). Abedon ST. ed. Bacteriophage Ecology: Population Growth, Evolution and Impact of Bacterial Viruses. Cambridge: Cambridge University Press. ISBN 0521858453.
- ^ Kutateladze M, Adamia R (August 2008). "Phage therapy experience at the Eliava Institute". Med Mal Infect 38 (8): 426'30. doi:10.1016/j.medmal.2008.06.023. PMID 18687542.
- ^ a b Merril CR, Scholl D, Adhya SL (June 2003). "The prospect for bacteriophage therapy in Western medicine". Nat Rev Drug Discov 2 (6): 489'97. doi:10.1038/nrd1111. PMID 12776223.
- ^ Abuladze T, Li M, Menetrez MY, Dean T, Senecal A, Sulakvelidze A (October 2008). "Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7". Appl. Environ. Microbiol. 74 (20): 6230'8. doi:10.1128/AEM.01465-08. PMID 18723643.
- ^ Thiel K (January 2004). "Old dogma, new tricks--21st Century phage therapy". Nat. Biotechnol. 22 (1): 31'6. doi:10.1038/nbt0104-31. PMID 14704699.
- ^ Parfitt T (2005). "Georgia: an unlikely stronghold for bacteriophage therapy". Lancet 365 (9478): 2166'7. doi:10.1016/S0140-6736(05)66759-1. PMID 15986542.
- ^ Lu TK, Collins JJ (2007). "Dispersing biofilms with engineered enzymatic bacteriophage". Proceedings of the National Academy of Sciences, USA 104 (27): 11197'11202. doi:10.1073/pnas.0704624104.
- ^ Williams SR, Gebhart D, Martin DW, Scholl D (2008). "Retargeting R-type pyocins to generate novel bactericidal protein complexes". Applied and Environmental Microbiology 74 (12): 3868'3876. doi:10.1128/AEM.00141-08. PMID 18441117.
- ^ Gillor O, Kirkup BC, Riley MA (2004). "Colicins and microcins: the next generation antimicrobials". Adv. Appl. Microbiol. 54: 129'46. doi:10.1016/S0065-2164(04)54005-4. PMID 15251279.
- ^ Kirkup BC (2006). "Bacteriocins as oral and gastrointestinal antibiotics: theoretical considerations, applied research, and practical applications". Curr. Med. Chem. 13 (27): 3335'50. doi:10.2174/092986706778773068. PMID 17168847.
- ^ Gillor O, Nigro LM, Riley MA (2005). "Genetically engineered bacteriocins and their potential as the next generation of antimicrobials". Curr. Pharm. Des. 11 (8): 1067'75. doi:10.2174/1381612053381666. PMID 15777256.
- ^ Jones RL, Peterson CM, Grady RW, Kumbaraci T, Cerami A, Graziano JH (1977). "Effects of iron chelators and iron overload on Salmonella infection". Nature 267 (5606): 63'65. doi:10.1038/267063a0. PMID 323727.
- ^ Brock JH, Liceaga J, Kontoghiorghes GJ (2006). "The effect of synthetic iron chelators on bacterial growth in human serum". FEMS Microbiology Letters 47 (1): 55'60. doi:10.1111/j.1574-6968.1988.tb02490.x.
- ^ Ibrahim AS, Edwards Jr JE, Fu Y, Spellberg B (2006). "Deferiprone iron chelation as a novel therapy for experimental mucormycosis". Journal of Antimicrobial Chemotherapy 58 (5): 1070'1073. doi:10.1093/jac/dkl350. PMID 16928702.
- ^ Soteriadou K, Papvassiliou P, Voyiatzaki C, Boelaert J (1995). Effects of iron chelation on the in-vitro growth of leishmania promastigotes. 35. pp. 23'29.
- ^ Nacar A, Nacar E (2008). "Phagotrophic protozoa: A new weapon against pathogens?". Medical Hypotheses 70 (1): 141'142. doi:10.1016/j.mehy.2007.03.037. PMID 17553625.
- ^ Ljungh A, Wadstrom T (editors) (2009). Lactobacillus Molecular Biology: From Genomics to Probiotics. Caister Academic Press. ISBN 978-1-904455-41-7.
[edit] External links
| Wikimedia Commons has media related to: Antibiotics |
- General Choice of Antibiotics Against Important Microorganisms
- Antibiotic at the Open Directory Project
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||
|
|||||||||||||||||
|
SOURCES.COM is an online portal and directory for journalists, news media, researchers and anyone seeking experts, spokespersons, and reliable information resources. Use SOURCES.COM to find experts, media contacts, news releases, background information, scientists, officials, speakers, newsmakers, spokespeople, talk show guests, story ideas, research studies, databases, universities, associations and NGOs, businesses, government spokespeople. Indexing and search applications by Ulli Diemer and Chris DeFreitas.
For information about being included in SOURCES as a expert or spokesperson see the FAQ . For partnerships, content and applications, and domain name opportunities contact us.