| Home | Sources Directory | News Releases | Calendar | Articles | | Contact | |
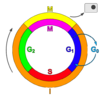
Cell cycle
The cell cycle, or cell-division cycle, is the series of events that takes place in a cell leading to its division and duplication (replication). In cells without a nucleus (prokaryotic), the cell cycle occurs via a process termed binary fission. In cells with a nucleus (eukaryotes), the cell cycle can be divided in two brief periods: interphase'during which the cell grows, accumulating nutrients needed for mitosis and duplicating its DNA'and the mitosis (M) phase, during which the cell splits itself into two distinct cells, often called "daughter cells". The cell-division cycle is a vital process by which a single-celled fertilized egg develops into a mature organism, as well as the process by which hair, skin, blood cells, and some internal organs are renewed.
Contents |
[edit] Phases
The cell cycle consists of four distinct phases: G1 phase, S phase (synthesis), G2 phase (collectively known as interphase) and M phase (mitosis). M phase is itself composed of two tightly coupled processes: mitosis, in which the cell's chromosomes are divided between the two daughter cells, and cytokinesis, in which the cell's cytoplasm divides in half forming distinct cells. Activation of each phase is dependent on the proper progression and completion of the previous one. Cells that have temporarily or reversibly stopped dividing are said to have entered a state of quiescence called G0 phase.

| State | Phase | Abbreviation | Description |
|---|---|---|---|
| quiescent/ senescent |
Gap 0 | G0 | A resting phase where the cell has left the cycle and has stopped dividing. |
| Interphase | Gap 1 | G1 | Cells increase in size in Gap 1. The G1 checkpoint control mechanism ensures that everything is ready for DNA synthesis. |
| Synthesis | S | DNA replication occurs during this phase. | |
| Gap 2 | G2 | During the gap between DNA synthesis and mitosis, the cell will continue to grow. The G2 checkpoint control mechanism ensures that everything is ready to enter the M (mitosis) phase and divide. | |
| Cell division | Mitosis | M | Cell growth stops at this stage and cellular energy is focused on the orderly division into two daughter cells. A checkpoint in the middle of mitosis (Metaphase Checkpoint) ensures that the cell is ready to complete cell division. |
After cell division, each of the daughter cells begin the interphase of a new cycle. Although the various stages of interphase are not usually morphologically distinguishable, each phase of the cell cycle has a distinct set of specialized biochemical processes that prepare the cell for initiation of cell division.
[edit] Resting (G0 phase)
The term "post-mitotic" is sometimes used to refer to both quiescent and senescent cells. Nonproliferative cells in multicellular eukaryotes generally enter the quiescent G0 state from G1 and may remain quiescent for long periods of time, possibly indefinitely (as is often the case for neurons). This is very common for cells that are fully differentiated. Cellular senescence is a state that occurs in response to DNA damage or degradation that would make a cell's progeny nonviable; it is often a biochemical alternative to the self-destruction of such a damaged cell by apoptosis.
[edit] Interphase
Before a cell can enter cell division, it needs to take in nutrients. All of the preparations are done during the interphase. Interphase proceeds in three stages, G1, S, and G2. Cell division operates in a cycle. Therefore, interphase is preceded by the previous cycle of mitosis and cytokinesis.
[edit] G1 phase
The first phase within interphase, from the end of the previous M phase until the beginning of DNA synthesis is called G1 (G indicating gap). It is also called the growth phase. During this phase the biosynthetic activities of the cell, which had been considerably slowed down during M phase, resume at a high rate. This phase is marked by synthesis of various enzymes that are required in S phase, mainly those needed for DNA replication. Duration of G1 is highly variable, even among different cells of the same species.[1]
[edit] S phase
The ensuing S phase starts when DNA synthesis commences; when it is complete, all of the chromosomes have been replicated, i.e., each chromosome has two (sister) chromatids. Thus, during this phase, the amount of DNA in the cell has effectively doubled, though the ploidy of the cell remains the same. Rates of RNA transcription and protein synthesis are very low during this phase. An exception to this is histone production, most of which occurs during the S phase.[2][3][4]
[edit] G2 phase
The cell then enters the G2 phase, which lasts until the cell enters mitosis. Again, significant biosynthesis occurs during this phase, mainly involving the production of microtubules, which are required during the process of mitosis. Inhibition of protein synthesis during G2 phase prevents the cell from undergoing mitosis.
[edit] Mitosis (M Phase)
The relatively brief M phase consists of nuclear division (karyokinesis). The M phase has been broken down into several distinct phases, sequentially known as:
- prophase,
- metaphase,
- anaphase,
- telophase
- cytokinesis (strictly speaking, cytokinesis is not part of mitosis but is an event that directly follows mitosis in which cytoplasm is divided into two daughter cells)
Mitosis is the process by which a eukaryotic cell separates the chromosomes in its cell nucleus into two identical sets in two nuclei.[5] It is generally followed immediately by cytokinesis, which divides the nuclei, cytoplasm, organelles and cell membrane into two cells containing roughly equal shares of these cellular components. Mitosis and cytokinesis together define the mitotic (M) phase of the cell cycle - the division of the mother cell into two daughter cells, genetically identical to each other and to their parent cell. This accounts for approximately 10% of the cell cycle.
Mitosis occurs exclusively in eukaryotic cells, but occurs in different ways in different species. For example, animals undergo an "open" mitosis, where the nuclear envelope breaks down before the chromosomes separate, while fungi such as Aspergillus nidulans and Saccharomyces cerevisiae (yeast) undergo a "closed" mitosis, where chromosomes divide within an intact cell nucleus.[6] Prokaryotic cells, which lack a nucleus, divide by a process called binary fission.
The process of mitosis is complex and highly regulated. The sequence of events is divided into phases, corresponding to the completion of one set of activities and the start of the next. These stages are prophase, prometaphase, metaphase, anaphase and telophase. During the process of mitosis the pairs of chromosomes condense and attach to fibers that pull the sister chromatids to opposite sides of the cell. The cell then divides in cytokinesis, to produce two identical daughter cells.[7]
Because cytokinesis usually occurs in conjunction with mitosis, "mitosis" is often used interchangeably with "orange phase". However, there are many cells where mitosis and cytokinesis occur separately, forming single cells with multiple nuclei. This occurs most notably among the fungi and slime moulds, but is found in various different groups. Even in animals, cytokinesis and mitosis may occur independently, for instance during certain stages of fruit fly embryonic development.[8] Errors in mitosis can either kill a cell through apoptosis or cause mutations that may lead to cancer.
[edit] Regulation of eukaryotic cell cycle
Regulation of the cell cycle involves processes crucial to the survival of a cell, including the detection and repair of genetic damage as well as the prevention of uncontrolled cell division. The molecular events that control the cell cycle are ordered and directional; that is, each process occurs in a sequential fashion and it is impossible to "reverse" the cycle.
[edit] Role of cyclins and CDKs
Two key classes of regulatory molecules, cyclins and cyclin-dependent kinases (CDKs), determine a cell's progress through the cell cycle.[9] Leland H. Hartwell, R. Timothy Hunt, and Paul M. Nurse won the 2001 Nobel Prize in Physiology or Medicine for their discovery of these central molecules.[10] Many of the genes encoding cyclins and CDKs are conserved among all eukaryotes, but in general more complex organisms have more elaborate cell cycle control systems that incorporate more individual components. Many of the relevant genes were first identified by studying yeast, especially Saccharomyces cerevisiae;[11] genetic nomenclature in yeast dubs many these genes cdc (for "cell division cycle") followed by an identifying number, e.g., cdc25 or cdc20.
Cyclins form the regulatory subunits and CDKs the catalytic subunits of an activated heterodimer; cyclins have no catalytic activity and CDKs are inactive in the absence of a partner cyclin. When activated by a bound cyclin, CDKs perform a common biochemical reaction called phosphorylation that activates or inactivates target proteins to orchestrate coordinated entry into the next phase of the cell cycle. Different cyclin-CDK combinations determine the downstream proteins targeted. CDKs are constitutively expressed in cells whereas cyclins are synthesised at specific stages of the cell cycle, in response to various molecular signals.[12]
[edit] General mechanism of cyclin-CDK interaction
| This section needs additional citations for verification. Please help improve this article by adding reliable references. Unsourced material may be challenged and removed. (July 2010) |
Upon receiving a pro-mitotic extracellular signal, G1 cyclin-CDK complexes become active to prepare the cell for S phase, promoting the expression of transcription factors that in turn promote the expression of S cyclins and of enzymes required for DNA replication. The G1 cyclin-CDK complexes also promote the degradation of molecules that function as S phase inhibitors by targeting them for ubiquitination. Once a protein has been ubiquitinated, it is targeted for proteolytic degradation by the proteasome.
Active S cyclin-CDK complexes phosphorylate proteins that make up the pre-replication complexes assembled during G1 phase on DNA replication origins. The phosphorylation serves two purposes: to activate each already-assembled pre-replication complex, and to prevent new complexes from forming. This ensures that every portion of the cell's genome will be replicated once and only once. The reason for prevention of gaps in replication is fairly clear, because daughter cells that are missing all or part of crucial genes will die. However, for reasons related to gene copy number effects, possession of extra copies of certain genes would also prove deleterious to the daughter cells.
Mitotic cyclin-CDK complexes, which are synthesized but inactivated during S and G2 phases, promote the initiation of mitosis by stimulating downstream proteins involved in chromosome condensation and mitotic spindle assembly. A critical complex activated during this process is a ubiquitin ligase known as the anaphase-promoting complex (APC), which promotes degradation of structural proteins associated with the chromosomal kinetochore. APC also targets the mitotic cyclins for degradation, ensuring that telophase and cytokinesis can proceed.
Interphase: Interphase generally lasts at least 12 to 24 hours in mammalian tissue. During this period, the cell is constantly synthesizing RNA, producing protein and growing in size. By studying molecular events in cells, scientists have determined that interphase can be divided into 4 steps: Gap 0 (G0), Gap 1 (G1), S (synthesis) phase, Gap 2 (G2).
[edit] Specific action of cyclin-CDK complexes
Cyclin D is the first cyclin produced in the cell cycle, in response to extracellular signals (e.g. growth factors). Cyclin D binds to existing CDK4, forming the active cyclin D-CDK4 complex. Cyclin D-CDK4 complex in turn phosphorylates the retinoblastoma susceptibility protein (Rb). The hyperphosphorylated Rb dissociates from the E2F/DP1/Rb complex (which was bound to the E2F responsive genes, effectively "blocking" them from transcription), activating E2F. Activation of E2F results in transcription of various genes like cyclin E, cyclin A, DNA polymerase, thymidine kinase, etc. Cyclin E thus produced binds to CDK2, forming the cyclin E-CDK2 complex, which pushes the cell from G1 to S phase (G1/S transition). Cyclin B along with cdc2 (cdc2 - fission yeasts (CDK1 - mammalia)) forms the cyclin B-cdc2 complex, which initiates the G2/M transition.[13] Cyclin B-cdc2 complex activation causes breakdown of nuclear envelope and initiation of prophase, and subsequently, its deactivation causes the cell to exit mitosis.[12]
[edit] Inhibitors
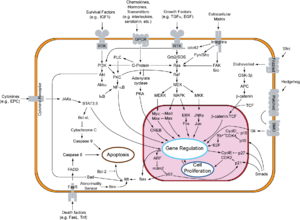
Two families of genes, the cip/kip family and the INK4a/ARF (Inhibitor of Kinase 4/Alternative Reading Frame) prevent the progression of the cell cycle. Because these genes are instrumental in prevention of tumor formation, they are known as tumor suppressors.
The cip/kip family includes the genes p21, p27 and p57. They halt cell cycle in G1 phase, by binding to, and inactivating, cyclin-CDK complexes. p21 is activated by p53 (which, in turn, is triggered by DNA damage e.g. due to radiation). p27 is activated by Transforming Growth Factor î� (TGF î�), a growth inhibitor.
The INK4a/ARF family includes p16INK4a, which binds to CDK4 and arrests the cell cycle in G1 phase, and p14arf which prevents p53 degradation.
Synthetic inhibitors of Cdc25 could also be useful for the arrest of cell cycle and therefore be useful as antineoplastic and anticancer agents.[14]
[edit] Checkpoints
Cell cycle checkpoints are used by the cell to monitor and regulate the progress of the cell cycle.[15] Checkpoints prevent cell cycle progression at specific points, allowing verification of necessary phase processes and repair of DNA damage. The cell cannot proceed to the next phase until checkpoint requirements have been met.
Several checkpoints are designed to ensure that damaged or incomplete DNA is not passed on to daughter cells. Two main checkpoints exist: the G1/S checkpoint and the G2/M checkpoint. G1/S transition is a rate-limiting step in the cell cycle and is also known as restriction point.[12] An alternative model of the cell cycle response to DNA damage has also been proposed, known as the postreplication checkpoint.
p53 plays an important role in triggering the control mechanisms at both G1/S and G2/M checkpoints.
[edit] Role in tumor formation
A disregulation of the cell cycle components may lead to tumor formation. As mentioned above, some genes like the cell cycle inhibitors, RB, p53 etc., when they mutate, may cause the cell to multiply uncontrollably, forming a tumor. Although the duration of cell cycle in tumor cells is equal to or longer than that of normal cell cycle, the proportion of cells that are in active cell division (versus quiescent cells in G0 phase) in tumors is much higher than that in normal tissue. Thus there is a net increase in cell number as the number of cells that die by apoptosis or senescence remains the same.
The cells which are actively undergoing cell cycle are targeted in cancer therapy as the DNA is relatively exposed during cell division and hence susceptible to damage by drugs or radiation. This fact is made use of in cancer treatment; by a process known as debulking, a significant mass of the tumor is removed which pushes a significant number of the remaining tumor cells from G0 to G1 phase (due to increased availability of nutrients, oxygen, growth factors etc.). Radiation or chemotherapy following the debulking procedure kills these cells which have newly entered the cell cycle.[12]
The fastest cycling mammalian cells in culture, and crypt cells in the intestinal epithelium, have a cycle time as short as 9 to 10 hours. Stem cells in resting mouse skin may have a cycle time of more than 200 hours. Most of this difference is due to the varying length of G1, the most variable phase of the cycle. M and S do not vary much.
In general, cells are most radiosensitive in late M and G2 phases and most resistant in late S.
For cells with a longer cell cycle time and a significantly long G1 phase, there is a second peak of resistance late in G1
The pattern of resistance and sensitivity correlates with the level of sulfhydryl compounds in the cell. Sulfhydryls are natural radioprotectors and tend to be at their highest levels in S and at their lowest near mitosis.
[edit] Synchronization of cell cultures
Several methods can be used to synchronise cell cultures by halting the cell cycle at a particular phase. For example, serum starvation[16] and treatment with thymidine or aphidicolin[17] halt the cell in the G1 phase, mitotic shake-off, treatment with colchicine[18] and treatment with nocodazole[19] halt the cell in M phase and treatment with 5-fluorodeoxyuridine halts the cell in S phase.
[edit] See also
- Cell cycle mathematical model
- Cell cycle analysis
- Mitosis
- Meiosis
- Interphase
- Autoradiography -- Used to determine the duration of each phase of the cell cycle.
- Biochemical Switches in the Cell Cycle
- Cdc25
[edit] References
- ^ Smith JA, Martin L (April 1973). "Do cells cycle?". Proc. Natl. Acad. Sci. U.S.A. 70 (4): 1263'7. doi:10.1073/pnas.70.4.1263. PMID 4515625.
- ^ Wu RS, Bonner WM (December 1981). "Separation of basal histone synthesis from S-phase histone synthesis in dividing cells". Cell 27 (2 Pt 1): 321'30. doi:10.1016/0092-8674(81)90415-3. PMID 7199388.
- ^ Nelson DM, Ye X, Hall C, Santos H, Ma T, Kao GD, Yen TJ, Harper JW, Adams PD (November 2002). "Coupling of DNA synthesis and histone synthesis in S phase independent of cyclin/cdk2 activity". Mol. Cell. Biol. 22 (21): 7459'72. doi:10.1128/MCB.22.21.7459-7472.2002. PMID 12370293.
- ^ Cameron IL, Greulich RC (July 1963). "Evidence for an essentially constant duration of DNA synthesis in renewing epithelia of the adult mouse". J. Cell Biol. 18: 31'40. doi:10.1083/jcb.18.1.31. PMID 14018040.
- ^ Rubenstein, Irwin, and Susan M. Wick. "Cell." World Book Online Reference Center. 2008. 12 January 2008 <http://www.worldbookonline.com/wb/Article?id=ar102240>
- ^ De Souza CP, Osmani SA (2007). "Mitosis, not just open or closed". Eukaryotic Cell 6 (9): 1521'7. doi:10.1128/EC.00178-07. PMID 17660363.
- ^ Maton, Anthea; Hopkins, Jean Johnson, Susan LaHart, David, Quon Warner, David, Wright, Jill D (1997). Cells: Building Blocks of Life. New Jersey: Prentice Hall. pp. 70'4. ISBN 0-13423476-6.
- ^ Lilly M, Duronio R (2005). "New insights into cell cycle control from the Drosophila endocycle". Oncogene 24 (17): 2765'75. doi:10.1038/sj.onc.1208610. PMID 15838513.
- ^ Nigg EA (June 1995). "Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle". Bioessays 17 (6): 471'80. doi:10.1002/bies.950170603. PMID 7575488.
- ^ "Press release". Nobelprize.org. http://nobelprize.org/nobel_prizes/medicine/laureates/2001/press.html.
- ^ Spellman PT, Sherlock G, Zhang MQ, Iyer VR, Anders K, Eisen MB, Brown PO, Botstein D, Futcher B (December 1998). "Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization". Mol. Biol. Cell 9 (12): 3273'97. PMID 9843569.
- ^ a b c d Robbins and Cotran; Kumar, Abbas, Fausto (2004). Pathological Basis of Disease. Elsevier. ISBN 81-8147-528-3.
- ^ Norbury C (1995). "Cdc2 protein kinase (vertebrates)". in Hardie, D. Grahame; Hanks, Steven. Protein kinase factsBook. Boston: Academic Press. pp. 184. ISBN 0-12-324719-5.
- ^ Presentation on CDC25 PHOSPHATASES: A Potential Target for Novel Anticancer Agents
- ^ Stephen J. Elledge (6 December 1996). "Cell Cycle Checkpoints: Preventing an Identity Crisis". Science 274 (5293): 1664'1672. doi:10.1126/science.274.5293.1664. PMID 8939848. http://www.sciencemag.org/cgi/content/abstract/274/5293/1664.
- ^ Kues WA, Anger M, Carnwath JW, Paul D, Motlik J, Niemann H (February 2000). "Cell cycle synchronization of porcine fetal fibroblasts: effects of serum deprivation and reversible cell cycle inhibitors". Biol. Reprod. 62 (2): 412'9. doi:10.1095/biolreprod62.2.412. PMID 10642581.
- ^ Pedrali-Noy G, Spadari S, Miller-Faurès A, Miller AO, Kruppa J, Koch G (January 1980). "Synchronization of HeLa cell cultures by inhibition of DNA polymerase alpha with aphidicolin". Nucleic Acids Res. 8 (2): 377'87. doi:10.1093/nar/8.2.377. PMID 6775308.
- ^ Prather RS, Boquest AC, Day BN (1999). "Cell cycle analysis of cultured porcine mammary cells". Cloning 1 (1): 17'24. doi:10.1089/15204559950020067. PMID 16218827.
- ^ Samaké S, Smith LC (October 1997). "Synchronization of cell division in eight-cell bovine embryos produced in vitro: effects of aphidicolin". Theriogenology 48 (6): 969'76. doi:10.1016/S0093-691X(97)00323-3. PMID 16728186.
[edit] Further reading
- Morgan DO (2007). The Cell Cycle: Principles of Control. London: Published by New Science Press in association with Oxford University Press. ISBN 0-87893-508-8.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2008). "Chapter 17". Molecular Biology of the Cell (5th ed.). New York: Garland Science. ISBN 978-0-8153-4111-6.
- Krieger M, Scott MP; Matsudaira PT, Lodish HF, Darnell JE, Zipursky L, Kaiser C; Berk A (2004). Molecular cell biology. New York: W.H. Freeman and CO. ISBN 0-7167-4366-3.
- Watson JD, Baker TA, Bell SP, Gann A, Levine M, Losick R (2004). "Chapter 7". Molecular biology of the gene (5th ed.). San Francisco: Pearson/Benjamin Cummings. ISBN 0-8053-4642-2.
[edit] External links
| Wikimedia Commons has media related to: Cell cycle |
 This article incorporates public domain material from the NCBI document "Science Primer".
This article incorporates public domain material from the NCBI document "Science Primer".
- Transcriptional program of the cell cycle: high-resolution timing
- Cell cycle and metabolic cycle regulated transcription in yeast
- Cell Cycle Animation 1Lec.com
- Cell Cycle and Cytokinesis - The Virtual Library of Biochemistry and Cell Biology
- Cell Cycle
- Cell Cycle Portal
- Fucci:Using GFP to visualize the cell-cycle
- Science Creative Quarterly's overview of the cell cycle
- Cells alive
- CCO The Cell-Cycle Ontology
- KEGG - Human Cell Cycle
- Cell cycle modeling
- Drosophila Cell Cycle Genes - The Interactive Fly
|
||||||||||||||||
|
||||||||||||||
|
SOURCES.COM is an online portal and directory for journalists, news media, researchers and anyone seeking experts, spokespersons, and reliable information resources. Use SOURCES.COM to find experts, media contacts, news releases, background information, scientists, officials, speakers, newsmakers, spokespeople, talk show guests, story ideas, research studies, databases, universities, associations and NGOs, businesses, government spokespeople. Indexing and search applications by Ulli Diemer and Chris DeFreitas.
For information about being included in SOURCES as a expert or spokesperson see the FAQ . For partnerships, content and applications, and domain name opportunities contact us.