
| Home | Sources Directory | News Releases | Calendar | Articles | | Contact | |
Cellulose
| Cellulose[1] | |
|---|---|
 |
|
 |
|
| Identifiers | |
| CAS number | 9004-34-6 |
| EC-number | 232-674-9 |
| Properties | |
| Molecular formula | (C6H10O5)n |
| Appearance | white powder |
| Density | 1.5 g/cm3 |
| Melting point |
decomp. |
| Solubility in water | none |
| Hazards | |
| EU Index | not listed |
| Related compounds | |
| Related compounds | Starch |
| Except where noted otherwise, data are given for materials in their standard state (at 25 –C, 100 kPa) |
|
| Infobox references | |
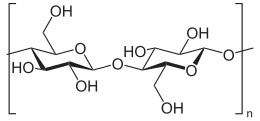
Cellulose is an organic compound with the formula (C6H10O5)n, a polysaccharide consisting of a linear chain of several hundred to over ten thousand î�(1'4) linked D-glucose units.[2][3]
Cellulose is the structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms. Cellulose is the most common organic compound on Earth. About 33 percent of all plant matter is cellulose (the cellulose content of cotton is 90 percent and that of wood is 40-50 percent).[4][5]
For industrial use, cellulose is mainly obtained from wood pulp and cotton. It is mainly used to produce paperboard and paper; to a smaller extent it is converted into a wide variety of derivative products such as cellophane and rayon. Converting cellulose from energy crops into biofuels such as cellulosic ethanol is under investigation as an alternative fuel source.
Some animals, particularly ruminants and termites, can digest cellulose with the help of symbiotic micro-organisms that live in their guts. Humans can digest cellulose to some extent,[6][7] however it is often referred to as 'dietary fiber' or 'roughage' (e.g. outer shell of Maize) and acts as a hydrophilic bulking agent for feces.
Contents |
[edit] History
Cellulose was discovered in 1838 by the French chemist Anselme Payen, who isolated it from plant matter and determined its chemical formula.[2][8] Cellulose was used to produce the first successful thermoplastic polymer, celluloid, by Hyatt Manufacturing Company in 1870. Hermann Staudinger determined the polymer structure of cellulose in 1920. The compound was first chemically synthesized (without the use of any biologically derived enzymes) in 1992, by Kobayashi and Shoda.[9]
[edit] Commercial products
Cellulose is the major constituent of paper, paperboard, and card stock and of textiles made from cotton, linen, and other plant fibers.
Cellulose can be converted into cellophane, a thin transparent film, and into rayon, an important fiber that has been used for textiles since the beginning of the 20th century. Both cellophane and rayon are known as "regenerated cellulose fibers"; they are identical to cellulose in chemical structure and are usually made from dissolving pulp via viscose. A more recent and environmentally friendly method to produce rayon is the Lyocell process. Cellulose is the raw material in the manufacture of nitrocellulose (cellulose nitrate) which was historically used in smokeless gunpowder and as the base material for celluloid used for photographic and movie films until the mid 1930s.
Cellulose is used to make water-soluble adhesives and binders such as methyl cellulose and carboxymethyl cellulose which are used in wallpaper paste. Microcrystalline cellulose (E460i) and powdered cellulose (E460ii) are used as inactive fillers in tablets[10] and as thickeners and stabilizers in processed foods. Cellulose powder is for example used in Kraft Parmesean Cheese to prevent caking inside the tube.
Cellulose is used in the laboratory as the stationary phase for thin layer chromatography. Cellulose fibers are also used in liquid filtration, sometimes in combination with diatomaceous earth or other filtration media, to create a filter bed of inert material. Cellulose is further used to make hydrophilic and highly absorbent sponges.
Cellulose insulation made from recycled paper is becoming popular as an environmentally preferable material for building insulation. It can be treated with boric acid as a fire retardant.
[edit] Cellulose source and energy crops
The major combustible component of non-food energy crops is cellulose, with lignin second. Non-food energy crops are more efficient than edible energy crops (which have a large starch component), but still compete with food crops for agricultural land and water resources.[11] Typical non-food energy crops include industrial hemp, switchgrass, Miscanthus, Salix (willow), and Populus (poplar) species.
Some bacteria can convert cellulose into ethanol which can then be used as a fuel; see cellulosic ethanol.

[edit] Structure and properties
Cellulose has no taste, is odourless, is hydrophilic, is insoluble in water and most organic solvents, is chiral and is biodegradable. It can be broken down chemically into its glucose units by treating it with concentrated acids at high temperature.
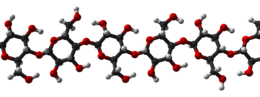
Cellulose is derived from D-glucose units, which condense through î�(1'4)-glycosidic bonds. This linkage motif contrasts with that for î�(1'4)-glycosidic bonds present in starch, glycogen, and other carbohydrates. Cellulose is a straight chain polymer: unlike starch, no coiling or branching occurs, and the molecule adopts an extended and rather stiff rod-like conformation, aided by the equatorial conformation of the glucose residues. The multiple hydroxyl groups on the glucose from one chain form hydrogen bonds with oxygen molecules on the same or on a neighbor chain, holding the chains firmly together side-by-side and forming microfibrils with high tensile strength. This strength is important in cell walls, where the microfibrils are meshed into a carbohydrate matrix, conferring rigidity to plant cells.
Compared to starch, cellulose is also much more crystalline. Whereas starch undergoes a crystalline to amorphous transition when heated beyond 60-70 –C in water (as in cooking), cellulose requires a temperature of 320 –C and pressure of 25 MPa to become amorphous in water.[12]
Several different crystalline structures of cellulose are known, corresponding to the location of hydrogen bonds between and within strands. Natural cellulose is cellulose I, with structures Iî� and Iî�. Cellulose produced by bacteria and algae is enriched in Iî� while cellulose of higher plants consists mainly of Iî�. Cellulose in regenerated cellulose fibers is cellulose II. The conversion of cellulose I to cellulose II is not reversible, suggesting that cellulose I is metastable and cellulose II is stable. With various chemical treatments it is possible to produce the structures cellulose III and cellulose IV.[13]
Many properties of cellulose depend on its chain length or degree of polymerization, the number of glucose units that make up one polymer molecule. Cellulose from wood pulp has typical chain lengths between 300 and 1700 units; cotton and other plant fibers as well as bacterial celluloses have chain lengths ranging from 800 to 10,000 units.[9] Molecules with very small chain length resulting from the breakdown of cellulose are known as cellodextrins; in contrast to long-chain cellulose, cellodextrins are typically soluble in water and organic solvents.
Plant-derived cellulose is usually contaminated with hemicellulose, lignin, pectin and other substances, while microbial cellulose is quite pure, has a much higher water content, and consists of long chains.
Cellulose is soluble in cupriethylenediamine (CED), cadmiumethylenediamine (Cadoxen), N-methylmorpholine N-oxide and lithium chloride / dimethylformamide[14]. This is used in the production of regenerated celluloses (as viscose and cellophane) from dissolving pulp.
[edit] Assaying cellulose
Given a cellulose-containing material, the carbohydrate portion that does not dissolve in a 17.5% solution of sodium hydroxide at 20 –C is î� cellulose, which is true cellulose. Acidification of the extract precipitates î� cellulose. The portion that dissolves in base but does not precipitate with acid is î� cellulose.
Cellulose can be assayed using a method described by Updegraff in 1969, where the fiber is dissolved in acetic and nitric acid to remove lignin, hemicellulose, and xylosans. The resulting cellulose is allowed to react with anthrone in sulfuric acid. The resulting coloured compound is assayed spectrophotometrically at a wavelength of approximately 635 nm.
In addition, cellulose is represented by the difference between acid detergent fiber (ADF) and acid detergent lignin (ADL).
[edit] Biosynthesis

In vascular plants cellulose is synthesized at the plasma membrane by rosette terminal complexes (RTCs). The RTCs are hexameric protein structures, approximately 25 nm in diameter, that contain the cellulose synthase enzymes that synthesise the individual cellulose chains.[15] Each RTC floats in the cell's plasma membrane and "spins" a microfibril into the cell wall.
RTCs contain at least three different cellulose synthases, encoded by CesA genes, in an unknown stoichiometry.[16] Separate sets of CesA genes are involved in primary and secondary cell wall biosynthesis.
Cellulose synthesis requires chain initiation and elongation, and the two processes are separate. CesA glucosyltransferase initiates cellulose polymerization using a steroid primer, sitosterol-beta-glucoside, and UDP-glucose.[17] Cellulose synthase utilizes UDP-D-glucose precursors to elongate the growing cellulose chain. A cellulase may function to cleave the primer from the mature chain.
[edit] Breakdown (cellulolysis)
Cellulolysis is the process of breaking down cellulose into smaller polysaccharides called cellodextrins or completely into glucose units; this is a hydrolysis reaction. Because cellulose molecules bind strongly to each other, cellulolysis is relatively difficult compared to the breakdown of other polysaccharides.[18]
Most mammals have only very limited ability to digest dietary fibres such as cellulose. Some ruminants like cows and sheep contain certain symbiotic anaerobic bacteria (like Cellulomonas) in the flora of the rumen, and these bacteria produce enzymes called cellulases that help the microorganism to break down cellulose; the breakdown products are then used by the bacteria for proliferation. The bacterial mass is later digested by the ruminant in its digestive system (stomach and small intestine). Similarly, lower termites contain in their hindguts certain flagellate protozoa which produce such enzymes; higher termites contain bacteria for the job. Some termites may also produce cellulase of their own.[19] Fungi, which in nature are responsible for recycling of nutrients, are also able to break down cellulose.
The enzymes utilized to cleave the glycosidic linkage in cellulose are glycoside hydrolases including endo-acting cellulases and exo-acting glucosidases. Such enzymes are usually secreted as part of multienzyme complexes that may include dockerins and cellulose binding modules.[20]
[edit] Hemicellulose
Hemicellulose is a polysaccharide related to cellulose that comprises ca. 20% of the biomass of most plants. In contrast to cellulose, hemicellulose is derived from several sugars in addition to glucose, especially xylose but also including mannose, galactose, rhamnose, and arabinose. Hemicellulose consists of shorter chains - around 200 sugar units. Furthermore, hemicellulose is branched, whereas cellulose is unbranched.
[edit] Derivatives
The hydroxyl groups (-OH) of cellulose can be partially or fully reacted with various reagents to afford derivatives with useful properties like mainly cellulose esters and cellulose ethers (-OR). In principle, though not always in current industrial practice, cellulosic polymers are renewable resources.
Ester derivatives include:
| Cellulose ester | Reagent | Example | Reagent | Group R |
|---|---|---|---|---|
| Organic esters | Organic acids | Cellulose acetate | Acetic acid and acetic anhydride | H or -(C=O)CH3 |
| Cellulose triacetate | Acetic acid and acetic anhydride | -(C=O)CH3 | ||
| Cellulose propionate | Propanoic acid | H or -(C=O)CH2CH3 | ||
| Cellulose acetate propionate | Acetic acid and propanoic acid | H or -(C=O)CH3 or -(C=O)CH2CH3 | ||
| Cellulose acetate butyrate | Acetic acid and butyric acid | H or -(C=O)CH3 or -(C=O)CH2CH2CH3 | ||
| Inorganic esters | Inorganic acids | Nitrocellulose (cellulose nitrate) | Nitric acid or another powerful nitrating agent |
The cellulose acetate and cellulose triacetate are film- and fiber-forming materials that find a variety of uses. The nitrocellulose was initially used as an explosive and was an early film forming material.
Ether derivatives include:
| Cellulose ethers | Reagent | Example | Reagent | Group R = H or | Water solubility | Application |
|---|---|---|---|---|---|---|
| Alkyl | Halogenoalkanes | Methylcellulose | Chloromethane | -CH3 | Cold water soluble | |
| Ethylcellulose | Chloroethane | -CH2CH3 | Water insoluble | A commercial thermoplastic used in coatings, inks, binders, and controlled-release drug tablets | ||
| Hydroxyalkyl | Epoxides | Hydroxyethyl cellulose | Ethylene oxide | -CH2CH2OH | Cold/hot water soluble | Gelling and thickening agent |
| Hydroxypropyl cellulose (HPC) | Propylene oxide | -CH2CH(OH)CH3 | Cold water soluble | |||
| Hydroxyethyl methyl cellulose | Chloromethane and ethylene oxide | -CH3 or -CH2CH2OH | Cold water soluble | Production of cellulose films | ||
| Hydroxypropyl methyl cellulose (HPMC) | Chloromethane and propylene oxide | -CH3 or -CH2CH(OH)CH3 | Cold water soluble | E464, Viscosity modifier, gelling, foaming and binding agent | ||
| Carboxyalkyl | Halogenated carboxylic acids | Carboxymethyl cellulose (CMC) | Chloroacetic acid | -CH2COOH | Cold/Hot water soluble | Often used as its sodium salt, sodium carboxymethyl cellulose (NaCMC). |
The sodium carboxymethyl cellulose can be cross-linked to give the croscarmellose sodium for use as a disintegrant in pharmaceutical formulations.
[edit] See also
[edit] References
- ^ Nishiyama, Yoshiharu; Langan, Paul; Chanzy, Henri (2002). "Crystal Structure and Hydrogen-Bonding System in Cellulose Iî� from Synchrotron X-ray and Neutron Fiber Diffraction". J. Am. Chem. Soc 124 (31): 9074'82. doi:10.1021/ja0257319. PMID 12149011..
- ^ a b Crawford, R. L. (1981). Lignin biodegradation and transformation. New York: John Wiley and Sons. ISBN 0-471-05743-6.
- ^ Updegraff DM (1969). "Semimicro determination of cellulose in biological materials". Analytical Biochemistry 32 (3): 420'424. doi:10.1016/S0003-2697(69)80009-6. PMID 5361396.
- ^ Cellulose. (2008). In Encyclopædia Britannica. Retrieved January 11, 2008, from Encyclopædia Britannica Online.
- ^ http://ipst.gatech.edu/faculty_new/faculty_bios/ragauskas/technical_reviews/Chemical%20Overview%20of%20Wood.pdf
- ^ Slavin, J.L., Brauer, P.M., and Marlett, J.A. (1980) "Neutral detergent fiber, hemicellulose and cellulose digestibility in human subjects." J Nutr 111(2):287-297 PMID 6257867
- ^ Joshi S, Agte V. (1995) Digestibility of dietary fiber components in vegetarian men. PMID 8719737
- ^ Young, Raymond (1986). Cellulose structure modification and hydrolysis. New York: Wiley. ISBN 0471827614.
- ^ a b Klemm, Dieter; Brigitte Heublein, Hans-Peter Fink, Andreas Bohn (2005). "Cellulose: Fascinating Biopolymer and Sustainable Raw Material". ChemInform 36 (36). doi:10.1002/chin.200536238.
- ^ Weiner, Myra L.; Lois A. Kotkoskie (1999). Excipient Toxicity and Safety. New York ; Dekker, c2000.. pp. 210. ISBN 0824782100, 9780824782108.
- ^ Holt-Gimenez, Eric 2007. Biofuels: Myths of the Agrofuels Transition. Backgrounder. Institute for Food and Development Policy, Oakland, CA. 13:2
- ^ Cooking cellulose in hot and compressed water Shigeru Deguchi, Kaoru Tsujii and Koki Horikoshi Chem. Commun., 2006, 3293 - 3295, doi:10.1039/b605812d
- ^ Structure and morphology of cellulose by Serge Pérez and William Mackie, CERMAV-CNRS, 2001. Chapter IV.
- ^ Stenius, Per (2000) "1" Forest Products Chemistry Papermaking Science and Technology 3 Finland: Fapet OY p. 35 ISBN 952-5216-03-9
- ^ Kimura, Laosinchai, Itoh, Cui, Linder, Brown, Plant Cell, 1999, 11, 2075-2085
- ^ Taylor, Howells, Huttly, Vickers, Turner, PNAS, 2003, 100, 1450-1455
- ^ Peng, Kawagoe, Hogan, Delmer, "Sitosterol-beta-glucoside as primer for cellulose synthesis in plants", Science, 2002, 295, 147-150. PMID 11778054
- ^ David G. Barkalow, Roy L. Whistler, "Cellulose", in AccessScience@McGraw-Hill, DOI 10.1036/1097-8542.118200. Retrieved 11 January 2008.
- ^ Tokuda, G; Watanabe, H (22 June 2007). "Hidden cellulases in termites: revision of an old hypothesis". Biology Letters 3 (3): 336'339. doi:10.1098/rsbl.2007.0073. PMID 17374589. PMC 2464699. http://rsbl.royalsocietypublishing.org/content/3/3/336.long
- ^ Br�¡s, Natércia; N. M. F. S. A. Cerqueira, P. A. Fernandes, M. J. Ramos (2008). "Carbohydrate Binding Modules from family 11: Understanding the binding mode of polysaccharides". International Journal of Quantum Chemistry 108 (11): 2030'2040. doi:10.1002/qua.21755.
[edit] External links
- Structure and morphology of cellulose by Serge Pérez and William Mackie, CERMAV-CNRS
- Cellulose, by Martin Chaplin, London South Bank University
- Clear description of a cellulose assay method at the Cotton Fiber Biosciences unit of the USDA.
- Cellulose films could provide flapping wings and cheap artificial muscles for robots - TechnologyReview.com
- Using cellulase enzymes in the bioethanol process
- A list of cellulolytic bacteria
|
|||||||||||||||||||||||||||||||||||||||||||||
|
SOURCES.COM is an online portal and directory for journalists, news media, researchers and anyone seeking experts, spokespersons, and reliable information resources. Use SOURCES.COM to find experts, media contacts, news releases, background information, scientists, officials, speakers, newsmakers, spokespeople, talk show guests, story ideas, research studies, databases, universities, associations and NGOs, businesses, government spokespeople. Indexing and search applications by Ulli Diemer and Chris DeFreitas.
For information about being included in SOURCES as a expert or spokesperson see the FAQ . For partnerships, content and applications, and domain name opportunities contact us.
