
| Home | Sources Directory | News Releases | Calendar | Articles | | Contact | |
Enantiomer
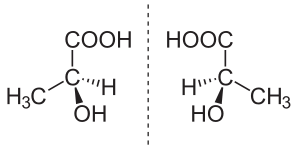
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are "non-superposable" (not identical), much as one's left and right hands are "the same" but opposite. [1] The term, pronounced /É�ˈnæntÉ�.É�mÉ�r/, is derived from the Greek 'á��î�î�î��„î�î¿ς', opposite, and 'î�î�ρî¿ς', part or portion.
Enantiopure compounds refer to samples having, within the limits of detection, molecules of only one chirality.[2]
Enantiomers have, when present in a symmetric environment, identical chemical and physical properties except for their ability to rotate plane-polarized light (+/-) by equal amounts but in opposite directions (nevertheless it's important to note that the polarized light can be considered an asymmetric media). A mixture of equal parts of an optically active isomer and its enantiomer is termed racemic and has a net rotation of plane-polarized light of zero.
Enantiomers of each other often show different chemical reactions with other substances that are also enantiomers. Since many molecules in the body of living beings are enantiomers themselves, there is often a marked difference in the effects of two enantiomers on living beings. In drugs, for example, the working substance is often one of two enantiomers, while the other one is responsible for adverse effects.
Contents |
[edit] Examples

An example of such an enantiomer is the sedative Thalidomide. It was sold in a number of countries across the world from 1957 until 1961 when it was withdrawn from the market after being found to be a cause of birth defects.
In the herbicide Mecoprop, the carboxyl group and the hydrogen atom on the central C-atom are exchanged (with the screen as plane of symmetry). After rotating one of the isomers 180 degrees (in the same plane), the two are still mirror images of each other. The mirror image of each enantiomer is superposable on the other enantiomer.
Another example are the antidepressant drugs Escitalopram (aka Lexapro) and Citalopram (aka Celexa). Citalopram is a racemate [1:1 mixture of (S)-Citalopram and (R)-Citalopram]; Escitalopram [(S)-Citalopram) is a pure enantiomer. The dosages for Escitalopram are typically 1/2 of those for Citalopram, and there are fewer side effects, suggesting that most side effects come from the (R)-enantiomer.[citation needed]
[edit] Enantioselective preparations
There are two main strategies for the preparation of enantiopure compounds. The first is known as chiral resolution. This method involves preparing the compound in racemic form, and separating it into its isomers. In his pioneering work, Louis Pasteur was able to isolate the isomers of tartaric acid because they crystallize from solution as crystals each with a different symmetry. A less common method is by enantiomer self-disproportionation.
The second strategy is asymmetric synthesis: the use of various techniques to prepare the desired compound in high enantiomeric excess. Techniques encompassed include the use of chiral starting materials (chiral pool synthesis), the use of chiral auxiliaries and chiral catalysts, and the application of asymmetric induction. The use of enzymes (biocatalysis) may also produce the desired compound.
Enantioconvergent synthesis is the synthesis of one enantiomer from a racemic precursor molecule utilizing both enantiomers. Thus, the two enantiomers of the reactant produce a single enantiomer of product.
[edit] Enantiopure medications
Advances in industrial chemical processes have made it economical for pharmaceutical manufacturers to take drugs that were originally marketed as a racemic mixture and market the individual enantiomers. In some cases, the enantiomers have genuinely different effects. In other cases, there may be no clinical benefit to the patient. Single-enantiomer drugs are separately patentable from the racemic mixture. It is possible that both enantiomers are active. Or, it may be that only one is active, in which case separating the mixture has no objective benefits, but extends the drug's patentability.[3]
[edit] See also
- Enantiopure drug
- Stereochemistry
- Dynamic stereochemistry
- Chirality (chemistry)
- Diastereomers
- Stereogenic
- Atropisomerism
- Antipode (chemistry)
[edit] References
- ^ International Union of Pure and Applied Chemistry. "enantiomer". Compendium of Chemical Terminology Internet edition.
- ^ International Union of Pure and Applied Chemistry. "enantiomerically pure (enantiopure)". Compendium of Chemical Terminology Internet edition.
- ^ Merrill Goozner (2004) (excerpt). The $800 Million Pill: The Truth Behind the Cost of New Drugs. University of California Press. ISBN 0-520-23945-8. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1395782#id973953.
|
|||||||||||||||||
|
SOURCES.COM is an online portal and directory for journalists, news media, researchers and anyone seeking experts, spokespersons, and reliable information resources. Use SOURCES.COM to find experts, media contacts, news releases, background information, scientists, officials, speakers, newsmakers, spokespeople, talk show guests, story ideas, research studies, databases, universities, associations and NGOs, businesses, government spokespeople. Indexing and search applications by Ulli Diemer and Chris DeFreitas.
For information about being included in SOURCES as a expert or spokesperson see the FAQ . For partnerships, content and applications, and domain name opportunities contact us.
